Pages from SimBasis appendix A property packages.pdf
- 文件大小: 280.98KB
- 文件类型: pdf
- 上传日期: 2025-08-23
- 下载次数: 0
概要信息:
Property Methods and Calculations A-1 A-1 A Property Methods and Calculations A.1 Selecting Property Methods ....................................................................... 3 A.2 Property Methods ........................................................................................ 8 A.2.1 Equations of State ................................................................................... 8 A.2.2 Activity Models....................................................................................... 15 A.2.3 Activity Model Vapour Phase Options.................................................... 33 A.2.4 Semi-Empirical Methods........................................................................ 34 A.2.5 Vapour Pressure Property Packages..................................................... 35 A.2.6 Miscellaneous - Special Application Methods........................................ 38 A.3 Enthalpy and Entropy Departure Calculations ....................................... 41 A.3.1 Equations of State ................................................................................. 42 A.3.2 Activity Models....................................................................................... 44 A.3.3 Lee-Kesler Option.................................................................................. 45 A.4 Physical and Transport Properties .......................................................... 48 A.4.1 Liquid Density ........................................................................................ 48 A.4.2 Vapour Density ...................................................................................... 49 A.4.3 Viscosity ................................................................................................ 49 A.4.4 Liquid Phase Mixing Rules for Viscosity ................................................ 51 A.4.5 Thermal Conductivity............................................................................. 52 A.4.6 Surface Tension ..................................................................................... 54 A.4.7 Heat Capacity ........................................................................................ 54 A.5 Volumetric Flow Rate Calculations .......................................................... 55 A.5.1 Available Flow Rates ............................................................................. 55 A.5.2 Liquid and Vapour Density Basis ........................................................... 56 A.5.3 Formulation of Flow Rate Calculations .................................................. 58 A.5.4 Volumetric Flow Rates as Specifications ............................................... 59 A-2 A-2 A.6 Flash Calculations..................................................................................... 60 A.6.1 T-P Flash Calculation............................................................................. 61 A.6.2 Vapour Fraction Flash............................................................................ 61 A.6.3 Enthalpy Flash ....................................................................................... 63 A.6.4 Entropy Flash......................................................................................... 63 A.6.5 Handling of Water .................................................................................. 63 A.6.6 Solids..................................................................................................... 65 A.6.7 Stream Information ................................................................................ 66 A.7 References ................................................................................................. 67 Property Methods and Calculations A-3 A-3 Introduction This appendix is organized such that the detailed calculations that occur within the Simulation Basis Manager and within the Flowsheet are explained in a logical manner. • In the first section, an overview of property method selection is presented. Various process systems and their recommended property methods are listed. • Detailed information is provided concerning each individual property method available in HYSYS. This section is further subdivided into equations of state, activity models, Chao- Seader based semi-empirical methods, vapour pressure models and miscellaneous methods. • Following the detailed property method discussion is the section concerning enthalpy and entropy departure calculations. The enthalpy and entropy options available within HYSYS are largely dependent upon your choice of a property method. • The physical and transport properties are covered in detail. The methods used by HYSYS in calculating liquid density, vapour density, viscosity, thermal conductivity and surface tension are listed. • HYSYS handles volume flow calculations in a unique way. To highlight the methods involved in calculating volumes, a separate section has been provided. • The next section ties all of the previous information together. Within HYSYS, the Flash calculation uses the equations of the selected property method, as well as the physical and transport property functions to determine all property values for Flowsheet streams. Once a flash calculation has been performed on an object, all of its thermodynamic, physical and transport properties are defined. The flash calculation in HYSYS does not require initial guesses or the specification of flash type to assist in its convergence. • A list of References is included at the end of the Appendix. A.1 Selecting Property Methods The property packages available in HYSYS allow you to predict properties of mixtures ranging from well defined light hydrocarbon systems to complex oil mixtures and highly non-ideal (non-electrolyte) chemical systems. HYSYS provides enhanced equations of state (PR and PRSV) for rigorous treatment of hydrocarbon systems; semi- empirical and vapour pressure models for the heavier hydrocarbon systems; steam correlations for accurate steam property predictions; A-4 Selecting Property Methods A-4 and activity coefficient models for chemical systems. All of these equations have their own inherent limitations and you are encouraged to become more familiar with the application of each equation. The following table lists some typical systems and recommended correlations. However, when in doubt of the accuracy or application of one of the property packages, contact Hyprotech to receive additional validation material or our best estimate of its accuracy. Type of System Recommended Property Method TEG Dehydration PR Sour Water PR, Sour PR Cryogenic Gas Processing PR, PRSV Air Separation PR, PRSV Atm Crude Towers PR, PR Options, GS Vacuum Towers PR, PR Options, GS (<10 mm Hg), Braun K10, Esso K Ethylene Towers Lee Kesler Plocker High H2 Systems PR, ZJ or GS (see T/P limits) Reservoir Systems PR, PR Options Steam Systems Steam Package, CS or GS Hydrate Inhibition PR Chemical systems Activity Models, PRSV HF Alkylation PRSV, NRTL (Contact Hyprotech) TEG Dehydration with Aromatics PR (Contact Hyprotech) Hydrocarbon systems where H2O solubility in HC is important Kabadi Danner Systems with select gases and light hydrocarbons MBWR Property Methods and Calculations A-5 A-5 For oil, gas and petrochemical applications, the Peng-Robinson EOS (PR) is generally the recommended property package. Hyprotech’s enhancements to this equation of state enable it to be accurate for a variety of systems over a wide range of conditions. It rigorously solves any single, two-phase or three-phase system with a high degree of efficiency and reliability, and is applicable over a wide range of conditions, as shown in the following table. The PR equation of state has been enhanced to yield accurate phase equilibrium calculations for systems ranging from low temperature cryogenic systems to high temperature, high pressure reservoir systems. The same equation of state satisfactorily predicts component distributions for heavy oil systems, aqueous glycol and CH3OH systems, and acid gas/sour water systems, although specific sour water models (Sour PR and Sour SRK) are available for more specialized treatment. Our high recommendation for the PR equation of state is largely due to the preferential attention that has been given to it by Hyprotech. Although the Soave-Redlich-Kwong (SRK) equation will also provide comparable results to the PR in many cases, it has been found that its range of application is significantly limited and it is not as reliable for non-ideal systems. For example, it should not be used for systems with CH3OH or glycols. As an alternate, the PRSV equation of state should also be considered. It can handle the same systems as the PR equation with equivalent, or better accuracy, plus it is more suitable for handling moderately non- ideal systems. The advantage of the PRSV equation is that not only does it have the potential to more accurately predict the phase behaviour of hydrocarbon systems, particularly for systems composed of dissimilar components, but it can also be extended to handle non-ideal systems with accuracies that rival traditional activity coefficient models. The only compromise is increased computational time and the additional interaction parameter that is required for the equation. The PR and PRSV equations of state perform rigorous three-phase flash calculations for aqueous systems containing H2O, CH3OH or glycols, as well as systems containing other hydrocarbons or non-hydrocarbons in the second liquid phase. For SRK, H2O is the only component that will initiate an aqueous phase. The Chao-Seader (CS) and Grayson-Streed Method Temp (°F) Temp (°C) Pressure (psia) Pressure (kPa) PR > -456 > -271 < 15,000 < 100,000 SRK > -225 > -143 < 5,000 < 35,000 NOTE: The range of applicability in many cases is more indicative of the availability of good data rather than on the actual limitations. A-6 Selecting Property Methods A-6 (GS) packages can also be used for three-phase flashes, but are restricted to the use of pure H2O for the second liquid phase. The PR can also be used for crude systems, which have traditionally been modelled with dual model thermodynamic packages (an activity model representing the liquid phase behaviour, and an equation of state or the ideal gas law for the vapour phase properties). These earlier models are suspect for systems with large amounts of light ends or when approaching critical regions. Also, the dual model system leads to internal inconsistencies. The proprietary enhancements to the PR and SRK methods allow these EOSs to correctly represent vacuum conditions and heavy components (a problem with traditional EOS methods), as well as handle the light ends and high-pressure systems. Activity Models, which handle highly non-ideal systems, are much more empirical in nature when compared to the property predictions in the hydrocarbon industry. Polar or non-ideal chemical systems have traditionally been handled using dual model approaches. In this type of approach, an equation of state is used for predicting the vapour fugacity coefficients and an activity coefficient model is used for the liquid phase. Since the experimental data for activity model parameters are fitted for a specific range, these property methods cannot be used as reliably for generalized application. The CS and GS methods, though limited in scope, may be preferred in some instances. For example, they are recommended for problems containing mainly liquid or vapour H2O because they include special correlations that accurately represent the steam tables. The Chao Seader method can be used for light hydrocarbon mixtures, if desired. The Grayson-Streed correlation is recommended for use with systems having a high concentration of H2 because of the special treatment given H2 in the development of the model. This correlation may also be slightly more accurate in the simulation of vacuum towers. The Vapour Pressure K models, Antoine, BraunK10 and EssoK models, are designed to handle heavier hydrocarbon systems at lower pressures. These equations have traditionally been applied for heavier hydrocarbon fractionation systems and consequently provide a good means of comparison against rigorous models. They should not be considered for VLE predictions for systems operating at high pressures or systems with significant quantities of light hydrocarbons. Property Methods and Calculations A-7 A-7 The Property Package methods in HYSYS are divided into basic categories, as shown in the following table. With each of the property methods listed are the available methods of VLE and Enthalpy/Entropy calculation. Please refer to Section A.3 - Enthalpy and Entropy Departure Calculations, for a description of Enthalpy and Entropy calculations. Property Method VLE Calculation Enthalpy/Entropy Calculation Equations of State PR PR PR PR LK ENTH PR Lee-Kesler SRK SRK SRK SRK LK ENTH SRK Lee-Kesler Kabadi Danner Kabadi Danner SRK Lee Kesler Plocker Lee Kesler Plocker Lee Kesler PRSV PRSV PRSV PRSV LK PRSV Lee-Kesler Sour PR PR & API-Sour PR SOUR SRK SRK & API-Sour SRK Zudkevitch-Joffee Zudkevitch-Joffee Lee-Kesler Activity Models Liquid Chien Null Chien Null Cavett Extended and General NRTL NRTL Cavett Margules Margules Cavett NRTL NRTL Cavett UNIQUAC UNIQUAC Cavett van Laar van Laar Cavett Wilson Wilson Cavett Vapour Ideal Gas Ideal Ideal Gas RK RK RK Virial Virial Virial Peng Robinson Peng Robinson Peng Robinson SRK SRK SRK Semi-Empirical Models Chao-Seader CS-RK Lee-Kesler Grayson-Streed GS-RK Lee-Kesler A-8 Property Methods A-8 A.2 Property Methods Details of each individual property method available in HYSYS will be provided in this section, including equations of state, activity models, Chao-Seader based empirical methods, vapour pressure models and miscellaneous methods. A.2.1 Equations of State HYSYS currently offers the enhanced Peng-Robinson1 (PR), and Soave- Redlich-Kwong2 (SRK) equations of state. In addition, HYSYS offers several methods which are modifications of these property packages, including PRSV, Zudkevitch Joffee (ZJ) and Kabadi Danner (KD). Lee Kesler Plocker3 (LKP) is an adaptation of the Lee Kesler equation for mixtures, which itself was modified from the BWR equation. Of these, the Peng-Robinson equation of state supports the widest range of operating conditions and the greatest variety of systems. The Peng- Robinson and Soave-Redlich-Kwong equations of state (EOS) generate all required equilibrium and thermodynamic properties directly. Although the forms of these EOS methods are common with other commercial simulators, they have been significantly enhanced by Hyprotech to extend their range of applicability. The Peng-Robinson property package options are PR, Sour PR, and PRSV. Soave-Redlich-Kwong equation of state options are the SRK, Sour SRK, KD and ZJ. Vapour Pressure Models Mod Antoine Mod Antoine-Ideal Gas Lee-Kesler Braun K10 Braun K10-Ideal Gas Lee-Kesler Esso K Esso-Ideal Gas Lee-Kesler Miscellaneous - Special Application Methods Amines Mod Kent Eisenberg (L), PR (V) Curve Fit Steam Packages ASME Steam ASME Steam Tables ASME Steam Tables NBS Steam NBS/NRC Steam Tables NBS/NRC Steam Tables MBWR Modified BWR Modified BWR Property Method VLE Calculation Enthalpy/Entropy Calculation It is important to note that the properties predicted by HYSYS’ PR and SRK equations of state will not necessarily agree with those predicted by the PR and SRK of other commercial simulators. Property Methods and Calculations A-9 A-9 PR and SRK The PR and SRK packages contain enhanced binary interaction parameters for all library hydrocarbon-hydrocarbon pairs (a combination of fitted and generated interaction parameters), as well as for most hydrocarbon-nonhydrocarbon binaries. For non-library or hydrocarbon pseudo components, HC-HC interaction parameters will be generated automatically by HYSYS for improved VLE property predictions. The PR equation of state applies a functionality to some specific component-component interaction parameters. Key components receiving special treatment include He, H2, N2, CO2, H2S, H2O, CH3OH, EG and TEG. For further information on application of equations of state for specific components, please contact Hyprotech. The following page provides a comparison of the formulations used in HYSYS for the PR and SRK equations of state. Note: The PR or SRK EOS should not be used for non- ideal chemicals such as alcohols, acids or other components. They are more accurately handled by the Activity Models (highly non- ideal) or the PRSV EOS (moderately non-ideal). Soave Redlich Kwong Peng Robinson where b= bi= a= ai= aci= αi 0.5 = P RT V b– ------------ a V V b+( ) ---------------------–= Z 3 Z 2 – A B– B 2 –( )Z AB–+ 0= P RT V b– ------------ a V V b+( ) b V b–( )+ ------------------------------------------------–= Z 3 1 B–( )Z 2 A 2B– 3B 2 –( )Z AB B 2 – B 3 –( )–+ + 0= xibi i 1= N ∑ xibi i 1= N ∑ 0.08664 RTci Pci ---------- 0.077796 RTci Pci ---------- xixj aiaj( )0.5 1 kij–( ) j 1= N ∑ i 1= N ∑ xixj aiaj( )0.5 1 kij–( ) j 1= N ∑ i 1= N ∑ aciαi aciαi 0.42748 RTci( )2 Pci ------------------ 0.457235 RTci( )2 Pci ------------------ 1 mi 1 Tri 0.5 –( )+ 1 mi 1 Tri 0.5 –( )+ A-10 Property Methods A-10 Kabadi Danner This KD4 model is a modification of the original SRK equation of State, enhanced to improve the vapour-liquid-liquid equilibria calculations for H2O-hydrocarbon systems, particularly in the dilute regions. The model is an improvement over previous attempts which were limited in the region of validity. The modification is based on an asymmetric mixing rule, whereby the interaction in the water phase (with its strong H2 bonding) is calculated based on both the interaction between the hydrocarbons and the H2O, and on the perturbation by hydrocarbon on the H2O-H2O interaction (due to its structure). Lee Kesler Plöcker Equation The Lee Kesler Plöcker equation is an accurate general method for non- polar substances and mixtures. Plöcker et al.3 applied the Lee Kesler equation to mixtures, which itself was modified from the BWR equation. mi= When an acentric factor > 0.49 is present HYSYS uses following corrected form: A= B= Soave Redlich Kwong Peng Robinson 0.48 1.574ωi 0.176ωi 2 –+ 0.37464 1.54226ωi 0.26992ωi 2 –+ 0.379642 1.48503 0.164423 1.016666ωi–( )ωi–( )ωi+ aP RT( )2 -------------- aP RT( )2 -------------- bP RT ------ bP RT ------ The Lee Kesler Plöcker equation does not use the COSTALD correlation in computing liquid density. This may result in differences when comparing results between equation of states. (A.1)z z o( ) ω ω r( )--------- z r( ) z o( ) –( )+= Property Methods and Calculations A-11 A-11 The compressibility factors are determined as follows: where: Mixing rules for pseudocritical properties are as follows: where: (A.2) (A.3) z pv RT ------ prvr Tr --------- z Tr vr Ak, ,( )= = = z 1 B vr ---- C vr 2 ---- D vr 5 ---- C4 Tr 3 vr 2 ---------- β γ vr 2 ----+ γ– vr 2 -----exp+ + + += vr pcv RTc --------= C c1 c2 Tr ----– c3 Tr 2 -----+= ω o( ) 0= B b1 b2 Tr ----– b3 Tr 2 -----– b4 Tr 3 -----–= D d1 d2 Tr ----–= ω r( ) 0.3978= (A.4)Tcm 1 Vcm η--------- xixjvcij j ∑ i ∑= Tcij Tci Tcj ( )1 2⁄ = Tcii Tci = Tcjj Tcj = vcm xixjvcij j ∑ i ∑= vcij 1 8 -- vci 1 3⁄ vcj 1 3⁄ +( ) 3 = vci zci RTci pci ----------= zci 0.2905 0.085ωi–= pcm zcm RTcm vcm -----------= zcm 0.2905 0.085ωm–= ωm xiωi i ∑= A-12 Property Methods A-12 Peng-Robinson Stryjek-Vera The Peng-Robinson Stryjek-Vera (PRSV) equation of state is a two-fold modification of the PR equation of state that extends the application of the original PR method for moderately non-ideal systems. It has been shown to match vapour pressures curves of pure components and mixtures more accurately than the PR method, especially at low vapour pressures. It has been successfully extended to handle non-ideal systems giving results as good as those obtained using excess Gibbs energy functions like the Wilson, NRTL or UNIQUAC equations. One of the proposed modifications to the PR equation of state by Stryjek and Vera was an expanded alpha, "α", term that became a function of acentricity and an empirical parameter, κi, used for fitting pure component vapour pressures. where: κ1i = characteristic pure component parameter ωi = acentric factor The adjustable κ1i term allows for a much closer fit of the pure component vapour pressure curves. This term has been regressed against the pure component vapour pressure for all components in HYSYS’ library. For pseudo components that have been generated to represent oil fractions, HYSYS will automatically regress the κ1i term for each pseudo component against the Lee-Kesler vapour pressure curves. For individual user-added hypothetical components, κ1i terms can either be entered or they will automatically be regressed against the Lee- Kesler, Gomez-Thodos or Reidel correlations. The second modification consists of a new set of mixing rules for mixtures. Conventional mixing rules are used for the volume and energy parameters in mixtures, but the mixing rule for the cross term, aij, is modified to adopt a composition dependent form. Although two different mixing rules were proposed in the original paper, HYSYS has (A.5) αi 1 κi 1 Tr 0.5 –( )+[ ] 2 = κi κ0i κ+ 1i 1 Tri 0.5 +( ) 0.7 Tri 0.5 –( )= κ0i 0.378893 1.4897153ωi 0.17131848ωi 2 – 0.0196554ωi 3 + += Note that if kij =kji , the mixing rules reduce to the standard PR equation of state. Property Methods and Calculations A-13 A-13 incorporated only the Margules expression for the cross term. where: Although only a limited number of binary pairs have been regressed for this equation, our limited experience suggests that the PRSV can be used to model moderately non-ideal systems such as H2O-alcohol systems, some hydrocarbon-alcohol systems. You can also model hydrocarbon systems with improved accuracy. Also, due to PRSV’s better vapour pressure predictions, improved heat of vaporization predictions should be expected. Sour Water Options The Sour option is available for both the PR and SRK equations of state. The Sour PR option combines the PR equation of state and Wilson’s API-Sour Model for handling sour water systems, while Sour SRK utilizes the SRK equation of state with the Wilson model. The Sour options use the appropriate equation of state for calculating the fugacities of the vapour and liquid hydrocarbon phases as well as the enthalpy for all three phases. The K-values for the aqueous phase are calculated using Wilson’s API-Sour method. This option uses Wilson’s model to account for the ionization of the H2S, CO2 and NH3 in the aqueous water phase. The aqueous model employs a modification of Van Krevelen’s original model with many of the key limitations removed. More details of the model are available in the original API publication 955 titled "A New Correlation of NH3, CO2, and H2S Volatility Data from Aqueous Sour Water Systems". The original model is applicable for temperatures between 20°C (68°F) and 140°C (285°F), and pressures up to 50 psi. Use of either the PR or SRK equation of state to correct vapour phase non idealities extends this range, but due to lack of experimental data, exact ranges cannot be specified. The acceptable pressure ranges for HYSYS' model vary depending upon the concentration of the acid gases and H2O. The method performs well when the H2O partial pressure is below 100 psi. This option may be applied to sour water strippers, hydrotreater loops, crude columns or any process containing hydrocarbons, acid gases and H2O. If the aqueous phase is not present, the method produces (A.6)aij aiiajj( )0.5 1.0 xikij– xjkji–( )= kij kji≠ Note that different values can be entered for each of the binary interaction parameters. It is important to note that because the method performs an ion balance for each K- value calculation, the flash calculation is much slower than the standard EOS. A-14 Property Methods A-14 identical results to the EOS, (PR or SRK depending on which option you have chosen). Zudkevitch Joffee The Zudkevitch Joffee model is a modification of the Redlich Kwong equation of state. This model has been enhanced for better prediction of vapour liquid equilibria for hydrocarbon systems, and systems containing H2. The major advantage of this model over the previous version of the RK equation is the improved capability of predicting pure component equilibria, and the simplification of the method for determining the required coefficients for the equation. Enthalpy calculations for this model will be performed using the Lee Kesler model. EOS Enthalpy Calculation With any the Equation of State options except ZJ and LKP, you can specify whether the Enthalpy will be calculated by either the Equation of State method or the Lee Kesler method. The ZJ and LKP must use the Lee Kesler method in Enthalpy calculations. Selection of an enthalpy method is done via radio buttons in the Enthalpy Method group. Selecting the Lee Kesler Enthalpy option results in a combined property package employing the appropriate equation of state (either PR or SRK) for vapour-liquid equilibrium calculations and the Lee- Kesler equation for calculation of enthalpies and entropies (for differences between EOS and LK methods, refer to the Section A.3 - Enthalpy and Entropy Departure Calculations). The LK method yields comparable results to HYSYS’ standard equations of state and has identical ranges of applicability. As such, this option with PR has a slightly greater range of applicability than with SRK. Figure A.1 The Lee-Kesler enthalpies may be slightly more accurate for heavy hydrocarbon systems, but require more computer resources because a separate model must be solved. Property Methods and Calculations A-15 A-15 Zero Kij Option HYSYS automatically generates hydrocarbon-hydrocarbon interaction parameters when values are unknown if the Estimate HC-HC/Set Non HC-HC to 0.0 radio button is selected. The Set All to 0.0 radio button turns off the automatic calculation of any estimated interaction coefficients between hydrocarbons. All binary interaction parameters that are obtained from the pure component library will remain. The Set All to 0.0 option may prove useful when trying to match results from other commercial simulators which may not supply interaction parameters for higher molecular weight hydrocarbons. A.2.2 Activity Models Although equation of state models have proven to be very reliable in predicting properties of most hydrocarbon based fluids over a large range of operating conditions, their application has been limited to primarily non-polar or slightly polar components. Polar or non-ideal chemical systems have traditionally been handled using dual model approaches. In this approach, an equation of state is used for predicting the vapour fugacity coefficients (normally ideal gas assumption or the Redlich Kwong, Peng-Robinson or SRK equations of state, although a Virial equation of state is available for specific applications) and an activity coefficient model is used for the liquid phase. Although there is considerable research being conducted to extend equation of state applications into the chemical arena (e.g., the PRSV equation), the state of the art of property predictions for chemical systems is still governed mainly by Activity Models. Activity Models are much more empirical in nature when compared to the property predictions (equations of state) typically used in the hydrocarbon industry. For example, they cannot be used as reliably as the equations of state for generalized application or extrapolating into untested operating conditions. Their tuning parameters should be fitted against a representative sample of experimental data and their application should be limited to moderate pressures. Consequently, more caution should be exercised when selecting these models for your simulation. Figure A.2 This option is set on the Binary Coeffs tab of the Fluid Package property view. A-16 Property Methods A-16 The phase separation or equilibrium ratio Ki for component i, defined in terms of the vapour phase fugacity coefficient and the liquid phase activity coefficient is calculated from the following expression: where: γi = liquid phase activity coefficient of component i fi° = standard state fugacity of component i P = system pressure φi = vapour phase fugacity coefficient of component i Although for ideal solutions the activity coefficient is unity, for most chemical (non-ideal) systems this approximation is incorrect. Dissimilar chemicals normally exhibit not only large deviations from an ideal solution, but the deviation is also found to be a strong function of the composition. To account for this non-ideality, activity models were developed to predict the activity coefficients of the components in the liquid phase. The derived correlations were based on the excess Gibbs energy function, which is defined as the observed Gibbs energy of a mixture in excess of what it would be if the solution behaved ideally, at the same temperature and pressure. For a multi-component mixture consisting of ni moles of component i, the total excess Gibbs free energy is represented by the following expression: where: γi is the activity coefficient for component i Activity Models produce the best results when they are applied in the operating region for which the interaction parameters were regressed. (A.7) Ki yi xi ---= γi fi° Pφi ---------= (A.8)G E RT ni γiln( )∑= Property Methods and Calculations A-17 A-17 The individual activity coefficients for any system can be obtained from a derived expression for excess Gibbs energy function coupled with the Gibbs-Duhem equation. The early models (Margules, van Laar) provide an empirical representation of the excess function that limits their application. The newer models such as Wilson, NRTL and UNIQUAC utilize the local composition concept and provide an improvement in their general application and reliability. All of these models involve the concept of binary interaction parameters and require that they be fitted to experimental data. Since the Margules and van Laar models are less complex than the Wilson, NRTL and UNIQUAC models, they require less CPU time for solving flash calculations. However, these are older and more empirically based models and generally give poorer results for strongly non-ideal mixtures such as alcohol-hydrocarbon systems, particularly for dilute regions. The Chien-Null model provides the ability to incorporate the different activity models within a consistent thermodynamic framework. Each binary can be represented by the model which best predicts its behaviour. The following table briefly summarizes recommended models for different applications (for a more detailed review, refer to the texts "The Properties of Gases & Liquids"8 and "Molecular Thermodynamics of Fluid Phase Equilibria" 9). A = Applicable; N/A = Not Applicable;? = Questionable; G = Good; LA = Limited Application Vapour phase non-ideality can be taken into account for each activity model by selecting the Redlich-Kwong, Peng-Robinson or SRK equations of state as the vapour phase model. When one of the equations of state is used for the vapour phase, the standard form of the Poynting correction factor is always used for liquid phase correction. If dimerization will occur in the vapour phase, the Virial equation of state should be selected as the vapour phase model. APPLICATION Margules van Laar Wilson NRTL UNIQUAC Binary Systems A A A A A Multicomponent Systems LA LA A A A Azeotropic Systems A A A A A Liquid-Liquid Equilibria A A N/A A A Dilute Systems ? ? A A A Self-Associating Systems ? ? A A A Polymers N/A N/A N/A N/A A Extrapolation ? ? G G G A-18 Property Methods A-18 The binary parameters required for the activity models have been regressed based on the VLE data collected from DECHEMA, Chemistry Data Series3. There are over 16,000 fitted binary pairs in the HYSYS library. The structures of all library components applicable for the UNIFAC VLE estimation are also in the library. The Poynting correction for the liquid phase is ignored if ideal solution behaviour is assumed. If you are using the built-in binary parameters, the ideal gas model should be used. All activity models, with the exception of the Wilson equation, can automatically calculate three phases given the correct set of energy parameters. The vapour pressures used in the calculation of the standard state fugacity are based on the pure component coefficients in HYSYS’ library using the modified form of the Antoine equation. When your selected components exhibit dimerization in the vapour phase, the Virial option should be selected as the vapour phase model. HYSYS contains fitted parameters for many carboxylic acids, and can estimate values from pure component properties if the necessary parameters are not available. Please refer to Section A.2.3 - Activity Model Vapour Phase Options for a detailed description of the Virial option. General Remarks The dual model approach for solving chemical systems with activity models cannot be used with the same degree of flexibility and reliability that the equations of state can be used for hydrocarbon systems. However, some checks can be devised to ensure a good confidence level in property predictions: • Check the property package selected for applicability for the system considered and see how well it matches the pure component vapour pressures. Although the predicted pure component vapour pressures should normally be acceptable, the parameters have been fitted over a large temperature range. Improved accuracies can be attained by regressing the parameters over the desired temperature range. • The automatic UNIFAC generation of energy parameters in HYSYS is a very useful tool and is available for all activity models. However, it must be used with caution. The standard fitted values in HYSYS will likely produce a better fit for the binary system than the parameters generated by UNIFAC. As a general rule, use the UNIFAC generated parameters only as a last resort. Please note that all of the binary parameters in HYSYS’ library have been regressed using an ideal gas model for the vapour phase. Note that HYSYS’ internally stored binary parameters have NOT been regressed against three phase equilibrium data. Property Methods and Calculations A-19 A-19 • Always use experimental data to regress the energy parameters when possible. The energy parameters in HYSYS have been regressed from experimental data, however, improved fits are still possible by fitting the parameters for the narrow operating ranges anticipated. The regressed parameters are based on data taken at atmospheric pressures. Exercise caution when extrapolating to higher or lower pressure (vacuum) applications. • Check the accuracy of the model for azeotropic systems. Additional fitting may be required to match the azeotrope with acceptable accuracy. Check not only for the temperature, but for the composition as well. • If three phase behaviour is suspected, additional fitting of the parameters may be required to reliably reproduce the VLLE equilibrium conditions. • An improvement in matching equilibrium data can be attained by including a temperature dependency of the energy parameters. However, depending on the validity or range of fit, this can lead to misleading results when extrapolating beyond the fitted temperature range. By default, HYSYS regresses ONLY the aij parameters while the bij parameters are set to zero, i.e., the aij term is assumed to be temperature independent. A temperature dependency can be incorporated by supplying a value for the bij term. The matrix for the bij values are displayed by choosing the Bij radio button to switch matrices (note the zero or blank entries for all the binary pairs). When using the NRTL, General NRTL or Extended NRTL equations, more than two matrices are available. In general, the second matrix is the Bij matrix, and the third matrix is the αij parameter where αij = αji. Any component pair with an aij value will have an associated α value. Immiscible This option is included for modelling the solubility of solutes in two coexisting liquid phases that are relatively immiscible with one another, such as a H2O-hydrocarbon system. In this system, the hydrocarbon components (solutes) are relatively insoluble in the water phase (solvent) whereas the solubility of the H2O in the hydrocarbon phase can become more significant. The limited mutual solubility behaviour can be taken into account when using any activity model with the exception of Wilson. This feature can be implemented for any single component pair by using the Immiscible radio button. Component i will be insoluble with component j, based on the highlighted cell location. Alternatively, you Please note that the activities for the unknown binaries are generated at pre-selected compositions and the supplied UNIFAC reference temperature. The Wilson equation does not support LLE equilibrium. A-20 Property Methods A-20 can have all j components treated as insoluble with component i. HYSYS will replace the standard binary parameters with those regressed specifically for matching the solubilities of the solutes in both phases. Note that both the aij and bij parameters are regressed with this option. These parameters were regressed from the mutual solubility data of n-C5, n-C6, n-C7, and n-C8 in H2O over a temperature range of 313 K to 473 K. The solubility of H2O in the hydrocarbon phase and the solubility of the hydrocarbons in the water phase will be calculated based on the fitted binary parameters regressed from the solubility data referenced above. Chien-Null The Chien Null model provides a consistent framework for applying existing activity models on a binary by binary basis. In this manner, the Chien Null model allows you to select the best activity model for each pair in the case. The Chien Null model allows 3 sets of coefficients for each component pair, accessible via the A, B and C coefficient matrices. Please refer to the following sections for an explanation of the terms for each of the models. Chien Null Form The Chien-Null generalized multi-component equation can be expressed as: Each of the parameters in this equation are defined specifically for each of the applicable activity methods. (A.9) 2 Γi L ln Aj i, xj j ∑ Aj i, xj j ∑ Aj i, xj j ∑ Aj i, xj j ∑ ------------------------------------------------------- xk Aj k, xj j ∑ Rj k, xj j ∑ Sj k, xj j ∑ Vj i, xj j ∑ ------------------------------------------------------------ ⋅ k ∑+= Ai k, Aj k, xj j ∑ ---------------------- Ri k, Rj k, xj j ∑ ---------------------- Si k, Sj k, xj j ∑ ---------------------– Vi k, Vj k, xj j ∑ ----------------------–+ Property Methods and Calculations A-21 A-21 Description of Terms The Regular Solution equation uses the following: δi is the solubility parameter in (cal/cm3)½ and vi L is the saturated liquid volume in cm3/mol calculated from: The van Laar, Margules and Scatchard Hamer use the following: For the van Laar, Margules and Scatchard Hamer equations: where: T must be in K. Note that this equation is of a different form than the original van Laar and Margules equations in HYSYS, which utilized an a + bT relationship. However, since HYSYS only contains aij values, the difference should not cause problems. (A.10)Ai j, vi L δi δj–( )2 RT ---------------------------= Ri j, Ai j, Aj i, --------= Vi j, Ri j,= Si j, Ri j,= (A.11)vi L vω i, 5.7 3Tr i,+( )= Model Ai,j Ri,j Si,j Vi,j van Laar Margules Scatchard Hamer γi j, ∞ ln Ai j, Aj i, -------- Ri j, Ri j, 2 γi j, ∞ ln 1 γi j, ∞ ln γj i, ∞ ln ---------------- + ------------------------------- Ai j, Aj i, -------- 1 1 2 γi j, ∞ ln 1 γi j, ∞ ln γj i, ∞ ln ---------------- + ------------------------------- Ai j, Aj i, -------- vi ∞ vj ∞----- vi ∞ vj ∞----- (A.12)γi j, ∞ ln ai j, bi j, T ------- cijT+ += If you have regressed parameters using HYPROP for any of the Activity Models supported under the Chien Null, they will not be read in. A-22 Property Methods A-22 The NRTL form for the Chien Null uses: The expression for the τ term under the Chien Null incorporates the R term of HYSYS’ NRTL into the values for aij and bij. As such, the values initialized for NRTL under Chien Null will not be the same as for the regular NRTL. When you select NRTL for a binary pair, aij will be empty (essentially equivalent to the regular NRTL bij term), bij will be initialized and cij will be the α term for the original NRTL, and will be assumed to be symmetric. The General Chien Null equation is: In all cases: With the exception of the Regular Solution option, all models can utilize 6 constants, ai,j, aj,i, bi,j, bj,i, ci,j and cj,i for each component pair. For all models, if the constants are unknown they can be estimated internally from the UNIFAC VLE or LLE methods, the Insoluble option, or using Henry’s Law coefficients for appropriate components. For the general Chien Null model, the cij’s are assumed to be 1. Extended and General NRTL The Extended and General NRTL models are variations of the NRTL model. More binary interaction parameters are used in defining the component activity coefficients. You may apply either model to systems: • with a wide boiling point range between components. • where you require simultaneous solution of VLE and LLE, and there exists a wide boiling point range or concentration range between components. (A.13)Ai j, 2τi j, Vi j,= Ri j, 1= Vi j, ci j,– τi j,( )exp= Si j, 1= τi j, ai j, bi j, T K( ) -----------+= (A.14) (A.15) Ai j, ai j, bi j, T K( ) -----------+= Ri j, Ai j, Aj i, --------= Vi j, Ci j,= Si j, Ci j,= Ai i, 0= Ri i, Si i, Vi i, 1= = = Property Methods and Calculations A-23 A-23 With the General NRTL model, you can specify the format for the Equations of τij and aij to be any of the following: Depending on which form of the equations that you have chosen, you will be able to specify values for the different component energy parameters. The General NRTL model provides radio buttons on the Binary Coeffs tab which access the matrices for the Aij, Bij, Cij, Fij, Gij, Alp1ij and Alp2ij energy parameters. τij and αij Options The equations options can be viewed in the Display Form drop down list on the Binary Coeffs tab of the Fluid Package property view. τij Aij Bij T ------ Cij T 2 ------ FijT Gij T( )ln+ + + += αij Alp1ij Alp2ijT+= τij Aij Bij T ------+ RT -------------------= αij Alp1ij= τij Aij Bij T ------ FijT Gij T( )ln+ + += αij Alp1ij Alp2ijT+= τij Aij Bijt Cij T ------+ += αij Alp1ij Alp2ijT+= where: T is in K and t is °C τij Aij Bij T ------+= αij Alp1ij= A-24 Property Methods A-24 The Extended NRTL model allows you to input values for the Aij, Bij, Cij, Alp1ij and Alp2ij energy parameters by selecting the appropriate radio button. You do not have a choice of equation format for τij and αij. The following is used: where: T is in K t is in °C Margules The Margules equation was the first Gibbs excess energy representation developed. The equation does not have any theoretical basis, but is useful for quick estimates and data interpolation. HYSYS has an extended multicomponent Margules equation with up to four adjustable parameters per binary. The four adjustable parameters for the Margules equation in HYSYS are the aij and aji (temperature independent) and the bij and bji terms (temperature dependent). The equation will use parameter values stored in HYSYS or any user supplied value for further fitting the equation to a given set of data. The Margules activity coefficient model is represented by the following equation: where: γi = activity coefficient of component i xi = mole fraction of component i (A.16) τij Aij Bijt Cij T ------+ + = αij Alp1ij Alp2ij+= The equation should not be used for extrapolation beyond the range over which the energy parameters have been fitted. (A.17)γiln 1.0 xi–[ ]2 Ai 2xi Bi Ai–( )+[ ]= Ai xj aij bijT+( ) 1.0 xi–( ) -------------------------- j 1= n ∑= Bi xj aji bjiT+( ) 1.0 xi–( ) -------------------------- j 1= n ∑= Property Methods and Calculations A-25 A-25 T = temperature (K) n = total number of components aij = non-temperature dependent energy parameter between components i and j bij = temperature dependent energy parameter between components i and j [1/K] aji = non-temperature dependent energy parameter between components j and i bji = temperature dependent energy parameter between components j and i [1/K] NRTL The NRTL (Non-Random-Two-Liquid) equation, proposed by Renon and Prausnitz in 1968, is an extension of the original Wilson equation. It uses statistical mechanics and the liquid cell theory to represent the liquid structure. These concepts, combined with Wilson’s local composition model, produce an equation capable of representing VLE, LLE and VLLE phase behaviour. Like the Wilson equation, the NRTL is thermodynamically consistent and can be applied to ternary and higher order systems using parameters regressed from binary equilibrium data. It has an accuracy comparable to the Wilson equation for VLE systems. The NRTL equation in HYSYS contains five adjustable parameters (temperature dependent and independent) for fitting per binary pair. The NRTL combines the advantages of the Wilson and van Laar equations, and, like the van Laar equation, it is not extremely CPU intensive and can represent LLE quite well. It is important to note that because of the mathematical structure of the NRTL equation, it can produce erroneous multiple miscibility gaps. Unlike the van Laar equation, NRTL can be used for dilute systems and hydrocarbon- alcohol mixtures, although it may not be as good for alcohol-hydrocarbon systems as the Wilson equation. A-26 Property Methods A-26 The NRTL equation in HYSYS has the following form: where: γi = activity coefficient of component i xi = mole fraction of component i T = temperature (K) n = total number of components aij = non-temperature dependent energy parameter between components i and j (cal/gmol) bij = temperature dependent energy parameter between components i and j (cal/gmol-K) αij = NRTL non-randomness constant for binary interaction note that αij = αji for all binaries The five adjustable parameters for the NRTL equation in HYSYS are the aij, aji, bij, bji, and αij terms. The equation will use parameter values stored in HYSYS or any user supplied value for further fitting the equation to a given set of data. UNIQUAC The UNIQUAC (UNIversal QUAsi Chemical) equation proposed by Abrams and Prausnitz in 1975 uses statistical mechanics and the quasi- chemical theory of Guggenheim to represent the liquid structure. The equation is capable of representing LLE, VLE and VLLE with accuracy comparable to the NRTL equation, but without the need for a non- randomness factor. The UNIQUAC equation is significantly more (A.18)γiln τjixjGji j 1= n ∑ xkGki k 1= n ∑ --------------------------- xjGij xkGkj k 1= n ∑ ----------------------- τij τmjxmGmj m 1= n ∑ xkGkj k 1= n ∑ -----------------------------------– j 1= n ∑+= Gij τijαij–[ ]exp= τij aij bijT+ RT ---------------------= Property Methods and Calculations A-27 A-27 detailed and sophisticated than any of the other activity models. Its main advantage is that a good representation of both VLE and LLE can be obtained for a large range of non-electrolyte mixtures using only two adjustable parameters per binary. The fitted parameters usually exhibit a smaller temperature dependence which makes them more valid for extrapolation purposes. The UNIQUAC equation utilizes the concept of local composition as proposed by Wilson. Since the primary concentration variable is a surface fraction as opposed to a mole fraction, it is applicable to systems containing molecules of very different sizes and shape, such as polymer solutions. The UNIQUAC equation can be applied to a wide range of mixtures containing H2O, alcohols, nitriles, amines, esters, ketones, aldehydes, halogenated hydrocarbons and hydrocarbons. HYSYS contains the following four-parameter extended form of the UNIQUAC equation. The four adjustable parameters for the UNIQUAC equation in HYSYS are the aij and aji terms (temperature independent), and the bij and bji terms (temperature dependent). The equation will use parameter values stored in HYSYS or any user supplied value for further fitting the equation to a given set of data. where: γi = activity coefficient of component i xi = mole fraction of component i T = temperature (K) n = total number of components (A.19)γiln Φi xi ----- ln 0.5Zqi θi Φi ----- Li θi Φi ----- Ljxj qi 1.0 θjτji j 1= n ∑ln– qi θjτij θkτkj k 1= n ∑ ---------------------- j 1= n ∑–+ j 1= n ∑–+ln+= A-28 Property Methods A-28 Lj = 0.5Z(rj-qj)-rj+1 Z = 10.0 co-ordination number aij = non-temperature dependent energy parameter between components i and j (cal/gmol) bij = temperature dependent energy parameter between components i and j (cal/gmol-K) qi = van der Waals area parameter - Awi /(2.5e9) Aw = van der Waals area ri = van der Waals volume parameter - Vwi /(15.17) Vw = van der Waals volume van Laar The van Laar equation was the first Gibbs excess energy representation with physical significance. The van Laar equation in HYSYS is a modified form of that described in "Phase Equilibrium in Process Design" by H.R. Null. This equation fits many systems quite well, particularly for LLE component distributions. It can be used for systems that exhibit positive or negative deviations from Raoult’s Law, however, it cannot predict maxima or minima in the activity coefficient. Therefore, it generally performs poorly for systems with halogenated hydrocarbons and alcohols. Due to the empirical nature of the equation, caution should be exercised in analyzing multi-component systems. It also has a tendency to predict two liquid phases when they do not exist. The van Laar equation has some advantages over the other activity models in that it requires less CPU time and can represent limited miscibility as well as three phase equilibrium. HYSYS uses the following extended, multi-component form of the van Laar equation. θi qixi qjxj∑---------------= τij aij bijT+ RT ---------------------–exp= The van Laar equation also performs poorly for dilute systems and CANNOT represent many common systems, such as alcohol- hydrocarbon mixtures, with acceptable accuracy. (A.20)γiln Ai 1.0 zi–[ ]2 1.0 Eizi+( )= Property Methods and Calculations A-29 A-29 where: γi = activity coefficient of component i xi = mole fraction of component i T = temperature (K) n = total number of components aij = non-temperature dependent energy parameter between components i and j bij = temperature dependent energy parameter between components i and j [1/K] aji = non-temperature dependent energy parameter between components j and i bji = temperature dependent energy parameter between components j and i [1/K] The four adjustable parameters for the van Laar equation in HYSYS are the aij, aji, bij, and bji terms. The equation will use parameter values stored in HYSYS or any user supplied value for further fitting the equation to a given set of data. Wilson The Wilson equation, proposed by Grant M. Wilson in 1964, was the first activity coefficient equation that used the local composition model to derive the Gibbs Excess energy expression. It offers a thermodynamically consistent approach to predicting multi- component behaviour from regressed binary equilibrium data. Our experience also shows that the Wilson equation can be extrapolated with reasonable confidence to other operating regions with the same set of regressed energy parameters. Ai xj aij bijT+( ) 1.0 xi–( ) -------------------------- j 1= n ∑= Bi xj aji bjiT+( ) 1.0 xi–( ) -------------------------- j 1= n ∑= Ei 4.0 if Ai Bi < 0.0, otherwise 0.0–= zi Aixi Aixi Bi 1.0 xi–( )+[ ] ------------------------------------------------= The Wilson equation CANNOT be used for problems involving liquid-liquid equilibrium. A-30 Property Methods A-30 Although the Wilson equation is more complex and requires more CPU time than either the van Laar or Margules equations, it can represent almost all non-ideal liquid solutions satisfactorily except electrolytes and solutions exhibiting limited miscibility (LLE or VLLE). It performs an excellent job of predicting ternary equilibrium using parameters regressed from binary data only. The Wilson equation will give similar results as the Margules and van Laar equations for weak non-ideal systems, but consistently outperforms them for increasingly non-ideal systems. The Wilson equation in HYSYS requires two to four adjustable parameters per binary. The four adjustable parameters for the Wilson equation in HYSYS are the aij and aji (temperature independent) terms, and the bij and bji terms (temperature dependent). Depending upon the available information, the temperature dependent parameters may be set to zero. Although the Wilson equation contains terms for temperature dependency, caution should be exercised when extrapolating. The Wilson activity model in HYSYS has the following form: where: γi = activity coefficient of component i xi = mole fraction of component i T = temperature (K) n = total number of components aij = non-temperature dependent energy parameter between components i and j (cal/gmol) bij = temperature dependent energy parameter between components i and j (cal/gmol-K) Note that setting all four parameters to zero does not reduce the binary to an ideal solution, but maintains a small effect due to molecular size differences represented by the ratio of molar volumes. (A.21)γiln 1.0 xjAij xkAki xjAkj j 1= n ∑ --------------------- k 1= n ∑– j 1= n ∑ln–= Aij Vj Vi ---- aij bijT+( ) RT --------------------------–exp= Property Methods and Calculations A-31 A-31 Vi = molar volume of pure liquid component i in m3/kgmol (litres/gmol) The equation will use parameter values stored in HYSYS or any user supplied value for further fitting the equation to a given set of data. Henry’s Law Henry’s Law cannot be selected explicitly as a property method in HYSYS. However, HYSYS will use Henry’s Law when an activity model is selected and "non-condensable" components are included within the component list. HYSYS considers the following components "non-condensable": The extended Henry’s Law equation in HYSYS is used to model dilute solute/solvent interactions. "Non-condensable" components are defined as those components that have critical temperatures below the temperature of the system you are modelling. The equation has the following form: where: i = solute or "non-condensable" component j = solvent or condensable component Component Simulation Name CH4 Methane C2H6 Ethane C2H4 Ethylene C2H2 Acetylene H2 Hydrogen He Helium Ar Argon N2 Nitrogen O2 Oxygen NO NO H2S H2S CO2 CO2 CO CO (A.22)Hijln A B T --- C T( ) DT+ln+ += A-32 Property Methods A-32 Hij = Henry’s coefficient between i and j in kPa A = A coefficient entered as aij in the parameter matrix B = B coefficient entered as aji in the parameter matrix C = C coefficient entered as bij in the parameter matrix D = D coefficient entered as bji in the parameter matrix T = temperature in degrees K An example of the use of Henry’s Law coefficients is illustrated below. The NRTL activity model is selected as the property method. There are three components in the Fluid Package, one of which, ethane, is a "non- condensable" component. On the Binary Coeffs tab of the Fluid Package property view, you can view the Henry’s Law coefficients for the interaction of ethane and the other components. By selecting the Aij radio button, you can view/edit the A and B coefficients. Choose the Bij radio button to enter or view the C and D coefficients in the Henry’s Law equation. If HYSYS does not contain pre-fitted Henry’s Law coefficients and Henry’s Law data is not available, HYSYS will estimate the missing coefficients. To estimate a coefficient (A or B in this case), select the Aij radio button, highlight a binary pair and press the Individual Pair button. The coefficients are regressed to fugacities calculated using the Chao-Seader/Prausnitz-Shair correlations for standard state fugacity and Regular Solution. To supply your own coefficients you must enter them directly into the Aij and Bij matrices, as shown previously. Figure A.3 HYSYS does not contain a pre-fitted Henry’s Law A coefficient for the ethane/ethanol pair. You can estimate it or provide your own value. Henry’s Law A coefficient for the interaction between C2 and H2O. Normal binary interaction coefficient for the H2O/Ethanol pair. C2 is a "non-condensable" component. Henry’s Law will be used for the interaction between C2 and the other components in the Fluid Package. Henry’s Law B coefficient for the interaction between C2 and H2O. Henry’s Law D coefficient for the interaction between C2 and H2O. Henry’s Law C coefficient for the interaction between C2 and H2O. Property Methods and Calculations A-33 A-33 No interaction between "non-condensable" component pairs is taken into account in the VLE calculations. A.2.3 Activity Model Vapour Phase Options There are several models available for calculating the Vapour Phase in conjunction with the selected liquid activity model. The choice will depend on specific considerations of your system. However, in cases when you are operating at moderate pressures (less than 5 atm), choosing Ideal Gas should be satisfactory. The choices are: Ideal The ideal gas law will be used to model the vapour phase. This model is appropriate for low pressures and for a vapour phase with little intermolecular interaction. Peng Robinson, SRK or RK To model non-idealities in the vapour phase, the PR, SRK or RK options can be used in conjunction with an activity model. The PR and SRK vapour phase models handle the same types of situations as the PR and SRK equations of state (refer to Section A.2.1 - Equations of State). When selecting one of these options (PR, SRK or RK) as the vapour phase model, you must ensure that the binary interaction parameters used for the activity model remain applicable with the chosen vapour model. You must keep in mind that all the binary parameters in the HYSYS Library have been regressed using the ideal gas vapour model. For applications where you have compressors or turbines being modelled within your Flowsheet, PR or SRK will be superior to either the RK or ideal vapour model. You will obtain more accurate horsepower values by using PR or SRK, as long as the light components within your Flowsheet can be handled by the selected vapour phase model (i.e. C2H4 or C3H6 are fine, but alcohols will not be modelled correctly). A-34 Property Methods A-34 Virial The Virial option enables you to better model vapour phase fugacities of systems displaying strong vapour phase interactions. Typically this occurs in systems containing carboxylic acids, or compounds that have the tendency to form stable H2 bonds in the vapour phase. In these cases, the fugacity coefficient shows large deviations from ideality, even at low or moderate pressures. HYSYS contains temperature dependent coefficients for carboxylic acids. You can overwrite these by changing the Association (ii) or Solvation (ij) coefficients from the default values.22 If the virial coefficients need to be calculated, HYSYS contains correlations utilizing the following pure component properties: • critical temperature • critical pressure • dipole moment • mean radius of gyration • association parameter • association parameter for each binary pair This option is restricted to systems where the density is moderate, typically less than one-half the critical density. The Virial equation used is valid for the following range: A.2.4 Semi-Empirical Methods The Chao-Seader10 and Grayson-Streed11 methods are older, semi- empirical methods. The GS correlation is an extension of the CS method with special emphasis on H2. Only the equilibrium results produced by these correlations is used by HYSYS. The Lee-Kesler method is used for liquid and vapour enthalpies and entropies as its results have been shown to be superior to those generated from the CS/ GS correlations. This method has also been adopted by and is recommended for use in the API Technical Data Book. (A.23)P T 2 -- yiPci i 1= m ∑ yiTci i 1= m ∑ --------------------≤ Property Methods and Calculations A-35 A-35 The following table gives an approximate range of applicability for these two methods, and under what conditions they are applicable. The GS correlation is recommended for simulating heavy hydrocarbon systems with a high H2 content, such as hydrotreating units. The GS correlation can also be used for simulating topping units and heavy ends vacuum applications. The vapour phase fugacity coefficients are calculated with the Redlich Kwong equation of state. The pure liquid fugacity coefficients are calculated via the principle of corresponding states. Modified acentric factors are included in HYSYS’ GS library for most components. Special functions have been incorporated for the calculation of liquid phase fugacities for N2, CO2 and H2S. These functions are restricted to hydrocarbon mixtures with less than five percent of each of the above components. As with the Vapour Pressure models, H2O is treated using a combination of the steam tables and the kerosene solubility charts from the API data book. This method of handling H2O is not very accurate for gas systems. Although three phase calculations are performed for all systems, it is important to note that the aqueous phase is always treated as pure H2O with these correlations. A.2.5 Vapour Pressure Property Packages Vapour pressure K value models may be used for ideal mixtures at low pressures. This includes hydrocarbon systems such as mixtures of ketones or alcohols where the liquid phase behaves approximately ideal. The models may also be used for first approximations for non- ideal systems. Method Temp. (°C) Temp. (°C) Press. (psia) Press. (kPa) CS 0 to 500 18 to 260 < 1500 < 10000 GS 0 to 800 18 to 425 < 3000 < 20000 Conditions of Applicability For all hydrocarbons (except CH4): 0.5 < Tri < 1.3 and Prmixture < 0.8 If CH4 or H2 is present: • molal average Tr < 0.93 • CH4 mole fraction < 0.3 • mole fraction dissolved gases < 0.2 When predicting K values for: Paraffinic or Olefinic Mixtures Aromatic Mixtures liquid phase aromatic mole fraction < 0.5 liquid phase aromatic mole fraction > 0.5 A-36 Property Methods A-36 Vapour pressures used in the calculation of the standard state fugacity are based on HYSYS’ library coefficients and a modified form of the Antoine equation. Vapour pressure coefficients for pseudo components may be entered or calculated from either the Lee-Kesler correlation for hydrocarbons, the Gomez-Thodos correlation for chemical compounds or the Reidel equation. The Vapour Pressure options include the Modified Antoine, BraunK10, and EssoK packages. Approximate ranges of application for each vapour pressure model are given below: Modified Antoine Vapour Pressure Model The modified Antoine equation assumes the form as set out in the DIPPR data bank. where A, B, C, D, E and F are fitted coefficients and the units of Pvap and T are kPa and K. These coefficients are available for all HYSYS library components. Vapour pressure coefficients for pseudo components may be entered or calculated from either the Lee-Kesler correlation for hydrocarbons, the Gomez-Thodos correlation for chemical compounds, or the Reidel equation. The Lee-Kesler model is used for enthalpy and entropy calculations for all vapour pressure models and all components with the exception of H2O, which is treated separately with the steam property correlation. All three phase calculations are performed assuming the aqueous phase is pure H2O and that H2O solubility in the hydrocarbon phase can be described using the kerosene solubility equation from the API data book (Figure 9A1.4). Because all of the Vapour Pressure options assume an ideal vapour phase, they are classified as Vapour Pressure Models. Model Temperature Press. (psia) Press. (kPa) Mod. Antoine < 1.6 Tci < 100 < 700 BraunK10 0°F (-17.78°C) < 1.6 Tci < 100 < 700 EssoK < 1.6 Tci < 100 < 700 (A.24)Pvapln A B T C+ ------------- D T ET F +ln+ += Note that all enthalpy and entropy calculations are performed using the Lee- Kesler model. Property Methods and Calculations A-37 A-37 This model is applicable for low pressure systems that behave ideally. For hydrocarbon components that you have not provided vapour pressure coefficients for, the model converts the Lee-Kesler vapour pressure model directly. As such, crude and vacuum towers can be modelled with this equation. When using this method for super-critical components, it is recommended that the vapour pressure coefficients be replaced with Henry’s Law coefficients. Changing Vapour Pressure coefficients can only be accomplished if your component is being installed as a Hypothetical. Braun K10 Model The Braun K10 model is strictly applicable to heavy hydrocarbon systems at low pressures. The model employs the Braun convergence pressure method, where, given the normal boiling point of a component, the K value is calculated at system temperature and 10 psia. The K10 value is then corrected for pressure using pressure correction charts. The K values for any components that are not covered by the charts are calculated at 10 psia using the modified Antoine equation and corrected to system conditions using the pressure correction charts. Accuracy suffers with this model if there are large amounts of acid gases or light hydrocarbons. All three phase calculations assume that the aqueous phase is pure H2O and that H2O solubility in the hydrocarbon phase can be described using the kerosene solubility equation from the API data book (Figure 9A1.4). Esso K Model The Esso Tabular model is strictly applicable to hydrocarbon systems at low pressures. The model employs a modification of the Maxwell- Bonnel vapour pressure model in the following format: The Lee-Kesler model is used for enthalpy and entropy calculations for all components with the exception of H2O which is treated with the steam tables. (A.25)Pvaplog Aix i∑= A-38 Property Methods A-38 where: Ai = fitted constants Tb i = normal boiling point corrected to K = 12 T = absolute temperature K = Watson characterisation factor For heavy hydrocarbon systems, the results will be comparable to the modified Antoine equation since no pressure correction is applied. For non-hydrocarbon components, the K value is calculated using the Antoine equation. Accuracy suffers if there is a large amount of acid gases or light hydrocarbons. All three phase calculations are performed assuming the aqueous phase is pure H2O and that H2O solubility in the hydrocarbon phase can be described using the kerosene solubility equation from the API data book (Figure 9A1.4). A.2.6 Miscellaneous - Special Application Methods Amines Property Package The amines package contains the thermodynamic models developed by D.B. Robinson & Associates for their proprietary amine plant simulator, called AMSIM. Their amine property package is available as an option with HYSYS giving you access to a proven third party property package for reliable amine plant simulation, while maintaining the ability to use HYSYS’ powerful flowsheeting capabilities. The chemical and physical property data base is restricted to amines and the following components: x i Tb i T ----- 0.0002867Tb i – 748.1 0.2145Tb i – -------------------------------------------= Note that the Lee-Kesler model is used for enthalpy and entropy calculations for all components with the exception of H2O which is treated with the steam tables. For the Amine property method, the vapour phase is modelled via the PR model. Component Class Specific Components Acid Gases CO2, H2S, COS, CS2 Hydrocarbons CH4 C7H16 Olefins C2=, C3= Mercaptans M-Mercaptan, E-Mercaptan Non Hydrocarbons H2, N2, O2, CO, H2O Property Methods and Calculations A-39 A-39 The equilibrium acid gas solubility and kinetic parameters for the aqueous alkanolamine solutions in contact with H2S and CO2 have been incorporated into their property package. The amines property package has been fitted to extensive experimental data gathered from a combination of D.B. Robinson’s in-house data, several unpublished sources, and numerous technical references. The following table gives the equilibrium solubility limitations that should be observed when using this property package. It is important to note that data have not been correlated for H2S and CO2 loadings greater than 1.0 mole acid gas/mole alkanolamine. The absorption of H2S and CO2 by aqueous alkanolamine solutions involves exothermic reactions. The heat effects are an important factor in amine treating processes and are properly taken into account in the amines property package. Correlations for the heats of solution are set up as a function of composition and amine type. The correlations were generated from existing published values or derived from solubility data using the Gibbs-Helmholtz equation. The amines package incorporates a specialized stage efficiency model to permit simulation of columns on a real tray basis. The stage efficiency model calculates H2S and CO2 component stage efficiencies based on the tray dimensions given and the calculated internal tower conditions for both absorbers and strippers. The individual component stage efficiencies are a function of pressure, temperature, phase compositions, flow rates, physical properties, mechanical tray design and dimensions as well as kinetic and mass transfer parameters. Since kinetic and mass transfer effects are primarily responsible for the H2S selectivity demonstrated by amine solutions, this must be accounted for by non unity stage efficiencies. See Chapter 7 - Column of the Steady State Modeling manual for details on how to specify or have HYSYS calculate the stage efficiencies. Note: this method does not allow any hypotheticals. Alkanolamine Alkanolamine Concentration (wt%) Acid Gas Partial Pressure (psia) Temperature (°F) Monoethanolamine, MEA 0 - 30 0.00001 - 300 77 - 260 Diethanolamine, DEA 0 - 50 0.00001 - 300 77 - 260 Triethanolamine, TEA 0 - 50 0.00001 - 300 77 - 260 Methyldiethanolamine, MDEA* 0 - 50 0.00001 - 300 77 - 260 Diglycolamine, DGA 50 - 70 0.00001 - 300 77 - 260 DIsoPropanolAmine, DIsoA 0 - 40 0.00001 - 300 77 - 260 * The amine mixtures, DEA/ MDEA and MEA/MDEA are assumed to be primarily MDEA, so use the MDEA value for these mixtures. A-40 Property Methods A-40 Steam Package HYSYS includes two steam packages: • ASME Steam • NBS Steam Both of these property packages are restricted to a single component, namely H2O. ASME Steam accesses the ASME 1967 steam tables. The limitations of this steam package are the same as those of the original ASME steam tables, i.e., pressures less than 15000 psia and temperatures greater than 32°F (0°C) and less than 1500°F. The basic reference is the book "Thermodynamic and Transport Properties of Steam" - The American Society of Mechanical Engineers - Prepared by C.A. Meyer, R.B. McClintock, G.J. Silvestri and R.C. Spencer Jr.20 Selecting NBS_Steam utilizes the NBS 1984 Steam Tables, which reportedly has better calculations near the Critical Point. MBWR In HYSYS, a 32-term modified BWR equation of state is used. The modified BWR may be written in the following form: where: F = exp (-0.0056 r2) (A.26)P RTρ NiXi i 1= 32 ∑+= X1 = ρ2T X8 = ρ3/T X15 = ρ6/T2 X22 = ρ5F/T2 X29 = ρ11F/T3 X2 = ρ2T1/2 X9 = ρ3/T2 X16 = ρ7/T X23 = ρ5F/T4 X30 = ρ13F/T2 X3 = ρ2 X10 = ρ4T X17 = ρ8/T X24 = ρ7F/T2 X31 = ρ13F/T3 X4 = ρ2/T X11 = ρ4 X18 = ρ8/T2 X25 = ρ7F/T3 X32 = ρ13F/T4 X5 = ρ2/T2 X12 = ρ4/T X19 = ρ9/T2 X26 = ρ9F/T2 X6 = ρ3T X13 = ρ5 X20 = ρ3F/T2 X27 = ρ9F/T4 X7 = ρ3 X14 = ρ6/T X21 = ρ3F/T3 X28 = ρ11F/T2 Property Methods and Calculations A-41 A-41 The modified BWR is applicable only for the following pure components: A.3 Enthalpy and Entropy Departure Calculations The Enthalpy and Entropy calculations are performed rigorously by HYSYS using the following exact thermodynamic relations: Component Temp. (K) Temp. (R) Max. Press. (MPa) Max. Press. (psia) Ar 84 - 400 151.2 - 720 100 14504 CH4 91 - 600 163.8 - 1080 200 29008 C2H4 104 - 400 187.2 - 720 40 5802 C2H6 90 - 600 162. - 1080 70 10153 C3H8 85 - 600 153. - 1080 100 14504 i-C4 114 - 600 205.2 - 1080 35 5076 n-C4 135 - 500 243. - 900 70 10153 CO 68 - 1000 122.4 - 1800 30 4351 CO2 217 - 1000 390.6 - 1800 100 14504 D2 29 - 423 52.2 - 761.4 320 46412 H2 14 - 400 25.2 - 720 120 17405 o-H2 14 - 400 25.2 - 720 120 17405 p-H2 14 - 400 25.2 - 720 120 17405 He 0.8 - 1500 1.4 - 2700 200 29008 N2 63 - 1900 113.4 - 3420 1000 145038 O2 54 - 400 97.2 - 720 120 17405 Xe 161 - 1300 289.8 - 2340 100 14504 Note that mixtures of different forms of H2 are also acceptable. The range of use for these components is shown in this table. Note that with semi-empirical and vapour pressure models, a pure liquid water phase will be generated and the solubility of H2O in the hydrocarbon phase will be determined from the kerosene solubility model. (A.27) (A.28) H H ID – RT ------------------- Z 1– 1 RT ------ T T∂ ∂P V P– Vd ∞ V ∫+= The Ideal Gas Enthalpy basis (HID) used by HYSYS is equal to the ideal gas Enthalpy of Formation at 25°C and 1 atm. S S° ID – RT ------------------ Zln P P° -----ln– 1 R -- T∂ ∂P V 1 V ---– Vd ∞ V ∫+= A-42 Enthalpy and Entropy Departure A-42 A.3.1 Equations of State For the Peng-Robinson Equation of State: where: For the SRK Equation of State: A and B term definitions are provided below: (A.29) (A.30) (A.31) The Ideal Gas Enthalpy basis (HID) used by HYSYS changes with temperature according to the coefficients on the TDep tab for each individual component. H H ID – RT ------------------- Z 1– 1 2 1.5 bRT ------------------- a T td da – V 2 0.5 1+( )b+ V 2 0.5 1–( )b+ ------------------------------------ ln–= S S° ID – R ------------------ Z B–( )ln P P° -----ln– A 2 1.5 bRT ------------------- T a -- td da V 2 0.5 1+( )b+ V 2 0.5 1–( )b+ ------------------------------------ ln–= a xixj aiaj( )0.5 1 kij–( ) j 1= N ∑ i 1= N ∑= (A.32) (A.33) H H ID – RT ------------------- Z 1– 1 bRT ---------- a T da dt -----– 1 b V --+ ln–= S S° ID – RT ------------------ Z b–( )ln P P° -----ln– A B --- T a -- da dt ----- 1 B Z ---+ ln+= Peng - Robinson Soave -Redlich - Kwong bi ai aci 0.077796 RTci Pci ---------- 0.08664 RTci Pci ---------- aciαi aciαi 0.457235 RTci( )2 Pci ------------------ 0.42748 RTci( )2 Pci ------------------ Property Methods and Calculations A-43 A-43 where: R = Ideal Gas constant H = Enthalpy S = Entropy subscripts: ID = Ideal Gas o = reference state PRSV The PRSV equation of state is an extension of the Peng-Robinson equation utilizing an extension of the κ expression as shown below: This results in the replacement of the αi term in the definitions of the A and B terms shown previously by the αi term shown above. mi Peng - Robinson Soave -Redlich - Kwong αi 1 mi 1 Tri 0.5 –( )+ 1 mi 1 Tri 0.5 –( )+ 0.37646 1.54226ωi 0.26992ωi 2 –+ 0.48 1.574ωi 0.176ωi 2 –+ a xixj aiaj( )0.5 1 kij–( ) j 1= N ∑ i 1= N ∑= (A.34) αi 1 κi 1 Tr 0.5 –( )+[ ] 2 = κi κ0i 1 Tri 0.5 +( ) 0.7 Tri–( )= κ0i 0.378893 1.4897153ωi 0.17131848ωi 2 – 0.0196554ωi 3 + += A-44 Enthalpy and Entropy Departure A-44 A.3.2 Activity Models The Liquid enthalpy and entropy for Activity Models is based on the Cavett Correlation: for Tri < 1: for Tri 1: where: where a1, a2, and a3 are functions of the Cavett parameter, fitted to match one known heat of vapourization. The Gas enthalpies and entropies are dependent on the model chosen to represent the vapour phase behaviour: Ideal Gas: (A.35) H L H ID – Tci ---------------------- max Hi°∆ L sb( ) Tci ------------------------- Hi°∆ L sb( ) Tci -------------------------, = ≥ (A.36) H L H ID – Tci ---------------------- max Hi°∆ L sb( ) Tci ------------------------- Hi°∆ L sp( ) Tci -------------------------, = (A.37) (A.38) Hi°∆ L sb( ) Tci ------------------------- a1 a2 1 Tri –( ) 1 a3 Tri 0.1–( )– += Hi°∆ L sp( ) Tci ------------------------- max 0 b1 b2Tri 2 b3Tri 3 b4Tri 4 b5Tri 2 + + + +,( )= (A.39) (A.40) H H ID = S S° ID Cv Td T ------------ T1 T2 ∫ R V2 V1 -----ln+= = Property Methods and Calculations A-45 A-45 Redlich-Kwong: Virial Equation: where: B = second virial coefficient of the mixture A.3.3 Lee-Kesler Option The Lee and Kesler method is an effort to extend the method originally proposed by Pitzer to temperatures lower than 0.8 Tr. Lee and Kesler expanded Pitzer’s method expressing the compressibility factor as: where: Z o = the compressibility factor of a simple fluid Z r = the compressibility factor of a reference fluid They chose the reduced form of the BWR equation of state to represent both Z o and Z r: (A.41) (A.42) H H ID – RT ------------------- Z 1– 1.5 bRT ---------- 1 b V ---+ ln–= S S° ID – RT ------------------ Z b–( )ln P P° -----ln– A 2B ------ 1 B Z ---+ ln+= (A.43) (A.44) H H ID – RT ------------------- T V B– ------------ dB dt ------– Z 1–( )+= S S° ID – R ------------------ RT V B– ------------ dB dT ------– R V V B– ------------ln– R V V° -----ln+= The SRK and PR are given in Section A.2.1 - Equations of State. (A.45)Z Z° ω ωr ----- Zr Z°–( )+= (A.46)Z 1 B Vr ----- C Vr 2 ----- D Vr 5 ----- D Tr 3 Vr 3 ----------- β γ Vr 2 -----– e γ Vr 2 ----- – + + + += A-46 Enthalpy and Entropy Departure A-46 where: The constants in these equations were determined using experimental compressibility and enthalpy data. Two sets of constants, one for the simple fluid (ωo = 0) and one for the reference fluid (ωr=0.3978, n-C8) were determined. The Enthalpy and Entropy departures are computed as follows: Vr VPc RTc ---------= B b1 b2 Tr ----– b3 Tr 2 -----– b4 Tr 4 -----–= C c1 c2 Tr ----– c3 Tr 3 -----+= D d1 d2 Tr ----+= (A.47) (A.48) (A.49) H H ID – RTc ------------------- Tr Z 1– b2 2 b3 Tr ---- 3 b4 Tr 2 -----+ + TrVr ------------------------------------– c2 3 c3 Tr 2 -----– 2TrVr 2 --------------------– d2 5TrVr 5 --------------– 3E+ = S S° ID – R ------------------ Zln P P° ----- ln– b1 b3 Tr 2 ----- 2 b4 Tr 3 -----+ + Vr ---------------------------------– c1 3 c3 Tr 2 -----– 2Vr 2 --------------------– d1 5Vr 2 --------– 2E+= E c4 2Tr 3γ ----------- β 1 β 1 γ Vr 2 -----+ + e γ Vr ---- – –+ = Property Methods and Calculations A-47 A-47 for mixtures, the Critical Properties are defined as follows: Fugacity Coefficient Soave-Redlich-Kwong Peng Robinson ω xiωi i 1= N ∑= zci 0.2905 0.0851ωi–= Vci Zci RTci Pci -----------------= Vc 1 8 -- xixj Vci 1 3 -- Vcj 1 3 -- + 3 j 1= N ∑ i 1= N ∑= Tc 1 8Vc -------- xixj Vci 1 3 -- Vcj 1 3 -- + 3 Tci Tcj ( )0.5 j 1= N ∑ i 1= N ∑= Pc 0.2905 0.085ω–( ) RTc Vc --------= (A.50)φiln Z Pb RT ------– ln– Z 1–( ) bi b --- a bRT ---------- 1 a -- 2ai 0.5 xjaj 0.5 1 kij–( ) j 1= N ∑ bi b ---– 1 b V --+ ln–+= (A.51)φiln Z Pb RT ------– ln– Z 1–( ) bi b --- a 2 1.5 bRT ------------------- 1 a -- 2ai 0.5 xjaj 0.5 1 kij–( ) j 1= N ∑ bi b ---– V 2 0.5 1+( )b+ V 2 0.5 1–( )b– ------------------------------------ln–+= A-48 Physical and Transport Properties A-48 A.4 Physical and Transport Properties The physical and transport properties that HYSYS calculates for a given phase are viscosity, density, thermal conductivity and surface tension. The models used for the transport property calculations have all been pre-selected to yield the best fit for the system under consideration. For example, the corresponding states model proposed by Ely and Hanley is used for viscosity predictions of light hydrocarbons (NBP<155), the Twu methodology for heavier hydrocarbons, and a modification of the Letsou-Stiel method for predicting the liquid viscosities of non-ideal chemical systems. A complete description of the models used for the prediction of the transport properties can be found in the references listed in each sub-section. All these models have been modified by Hyprotech to improve the accuracy of the correlations. In the case of multiphase streams, the transport properties for the mixed phase are meaningless and are reported as, although the single phase properties are known. There is an exception with the pipe and heat exchanger operations. For three-phase fluids, HYSYS uses empirical mixing rules to determine the apparent properties for the combined liquid phases. A.4.1 Liquid Density Saturated liquid volumes are obtained using a corresponding states equation developed by R. W. Hankinson and G. H. Thompson13 which explicitly relates the liquid volume of a pure component to its reduced temperature and a second parameter termed the characteristic volume. This method has been adopted as an API standard. The pure compound parameters needed in the corresponding states liquid density (COSTALD) calculations are taken from the original tables published by Hankinson and Thompson, and the API data book for components contained in HYSYS’ library. The parameters for hypothetical components are based on the API gravity and the generalized Lu equation. Although the COSTALD method was developed for saturated liquid densities, it can be applied to sub-cooled liquid densities, i.e., at pressures greater than the vapour pressure, using the Chueh and Prausnitz correction factor for compressed fluids. It is used to predict the density for all systems whose pseudo-reduced temperature is below 1.0. Above this temperature, the equation of state compressibility factor is used to calculate the liquid density. Property Methods and Calculations A-49 A-49 Pseudo components generated in the Oil Characterization Environment will have their densities either calculated from internal correlations or generated from input curves. Given a bulk density, the densities of the pseudo components are adjusted such that: The characteristic volume for each pseudo component is calculated using the adjusted densities and the physical properties. The calculated characteristic volumes are then adjusted such that the bulk density calculated from the COSTALD equation matches the density calculated via above equation. This ensures that a given volume of fluid will contain the same mass whether it is calculated with the sum of the component densities or the COSTALD equation. A.4.2 Vapour Density The density for all vapour systems at a given temperature and pressure is calculated using the compressibility factor given by the equation of state or by the appropriate vapour phase model for Activity Models. A.4.3 Viscosity HYSYS will automatically select the model best suited for predicting the phase viscosities of the system under study. The model selected will be from one of the three available in HYSYS: a modification of the NBS method (Ely and Hanley), Twu’s model, or a modification of the Letsou- Stiel correlation. HYSYS will select the appropriate model using the following criteria: All of the models are based on corresponding states principles and have been modified for more reliable application. Internal validation showed that these models yielded the most reliable results for the chemical systems shown. Viscosity predictions for light hydrocarbon liquid phases and vapour phases were found to be handled more (A.52) ρbulk 1.0 xi ρi° ------∑ -------------= Chemical System Vapour Phase Liquid Phase Lt Hydrocarbons (NBP<155°F) Mod Ely & Hanley Mod Ely & Hanley Hvy Hydrocarbons (NBP>155°F) Mod Ely & Hanley Twu Non-Ideal Chemicals Mod Ely & Hanley Mod Letsou-Stiel A-50 Physical and Transport Properties A-50 reliably by an in-house modification of the original Ely and Hanley model, heavier hydrocarbon liquids were more effectively handled by Twu’s model, and chemical systems were more accurately handled by an in-house modification of the original Letsou-Stiel model. A complete description of the original corresponding states (NBS) model used for viscosity predictions is presented by Ely and Hanley in their NBS publication. The original model has been modified to eliminate the iterative procedure for calculating the system shape factors. The generalized Leech-Leland shape factor models have been replaced by component specific models. HYSYS constructs a PVT map for each component using the COSTALD for the liquid region. The shape factors are adjusted such that the PVT map can be reproduced using the reference fluid. The shape factors for all the library components have already been regressed and are included in the Pure Component Library. Pseudo component shape factors are regressed using estimated viscosities. These viscosity estimations are functions of the pseudo component Base Properties and Critical Properties. Pseudo components generated in the Oil Characterization Environment have the additional ability of having their shape factors regressed to match kinematic or dynamic viscosity assays. The general model employs CH4 as a reference fluid and is applicable to the entire range of non-polar fluid mixtures in the hydrocarbon industry. Accuracy for highly aromatic or naphthenic crudes will be increased by supplying viscosity curves when available, since the pure component property generators were developed for average crude oils. The model also handles H2O and acid gases as well as quantum gases. Although the modified NBS model handles these systems very well, the Twu method was found to do a better job of predicting the viscosities of heavier hydrocarbon liquids. The Twu model9 is also based on corresponding states principles, but has implemented a viscosity correlation for n-alkanes as its reference fluid instead of CH4. A complete description of this model is given in the paper entitled "Internally Consistent Correlation for Predicting Liquid Viscosities of Petroleum Fractions"15. For chemical systems the modified NBS model of Ely and Hanley is used for predicting vapour phase viscosities, whereas a modified form of the Letsou-Stiel model is used for predicting the liquid viscosities. This method is also based on corresponding states principles and was found to perform satisfactorily for the components tested. Property Methods and Calculations A-51 A-51 The shape factors contained in the HYSYS Pure Component Library have been fit to match experimental viscosity data over a broad operating range. Although this will yield good viscosity predictions as an average over the entire range, improved accuracy over a narrow operating range can be achieved by using the Tabular features (see Chapter 1 - Fluid Package for more information). A.4.4 Liquid Phase Mixing Rules for Viscosity The estimates of the apparent liquid phase viscosity of immiscible Hydrocarbon Liquid - Aqueous mixtures are calculated using the following "mixing rules": • If the volume fraction of the hydrocarbon phase is greater than or equal to 0.5, the following equation is used17: where: µeff = apparent viscosity µoil = viscosity of Hydrocarbon phase νoil = volume fraction Hydrocarbon phase • If the volume fraction of the hydrocarbon phase is less than 0.33, the following equation is used18: where: µeff = apparent viscosity µoil = viscosity of Hydrocarbon phase µH2O= viscosity of Aqueous phase νoil = volume fraction Hydrocarbon phase • If the volume of the hydrocarbon phase is between 0.33 and 0.5, the effective viscosity for combined liquid phase is calculated using a weighted average between Equation (A.53) and Equation (A.54). (A.53)µeff µoile 3.6 1 νoil–( ) = (A.54)µeff 1 2.5νoil µoil 0.4µH2O+ µoil µH2O+ ----------------------------------- + µH2O= A-52 Physical and Transport Properties A-52 The remaining properties of the pseudo phase are calculated as follows: A.4.5 Thermal Conductivity As in viscosity predictions, a number of different models and component specific correlations are implemented for prediction of liquid and vapour phase thermal conductivities. The text by Reid, Prausnitz and Poling16 was used as a general guideline in determining which model was best suited for each class of components. For hydrocarbon systems the corresponding states method proposed by Ely and Hanley14 is generally used. The method requires molecular weight, acentric factor and ideal heat capacity for each component. These parameters are tabulated for all library components and may either be input or calculated for hypothetical components. It is recommended that all of these parameters be supplied for non- hydrocarbon hypotheticals to ensure reliable thermal conductivity coefficients and enthalpy departures. The modifications to the method are identical to those for the viscosity calculations. Shape factors calculated in the viscosity routines are used directly in the thermal conductivity equations. The accuracy of the method will depend on the consistency of the original PVT map. The Sato-Reidel method16 is used for liquid phase thermal conductivity predictions of glycols and acids, the Latini et al. method16 is used for esters, alcohols and light hydrocarbons in the range of C3 - C7, and the Missenard and Reidel method16 is used for the remaining components. For vapour phase thermal conductivity predictions, the Misic and Thodos, and Chung et al.16 methods are used. The effect of higher pressure on thermal conductivities is taken into account by the Chung et al. method. As with viscosity, the thermal conductivity for two liquid phases is approximated by using empirical mixing rules for generating a single pseudo liquid phase property. The thermal conductivity for an (A.55) MWeff xiMWi∑= ρeff 1 xi ρ i ---- ∑ -----------------= Cpeff xiCpi∑= (molecular weight) (mixture density) (mixture specific heat) Property Methods and Calculations A-53 A-53 immiscible binary of liquid phases is calculated by the following equation21: where: λLmix = mixture liquid thermal conductivity at temperature T(K) κij = liquid thermal conductivity of pure component i or j at temperature T λL1 = liquid thermal conductivity of liquid phase 1 λL2 = liquid thermal conductivity of liquid phase 2 φ1 = φ2 = xi = mole fraction of component i Vi = molar volume of component i (A.56)λLmix φ1 2λL1 2φ1φ2λ12 φ2 2λL2 + += λLmix φiφjkij j ∑ i ∑= kij 2 1 ki⁄( ) 1 kj⁄( )+ -------------------------------------= x1V1 xiVi i 1= 2 ∑ ------------------- x2V2 xiVi i 1= 2 ∑ ------------------- A-54 Physical and Transport Properties A-54 A.4.6 Surface Tension Surface tensions for hydrocarbon systems are calculated using a modified form of the Brock and Bird equation8. The equation expresses the surface tension, σ, as a function of the reduced and critical properties of the component. The basic form of the equation was used to regress parameters for each family of components. where: σ = surface tension (dynes/cm2) Q = 0.1207[1.0 + TBR ln Pc /(1.0 - TBR)] - 0.281 a = parameter fitted for each chemical class b = c0 + c1 ω + c2 ω2 + c3 ω3 (parameter fitted for each chemical class, expanded as a polynomial in acentricity) For aqueous systems, HYSYS employs a polynomial to predict the surface tension. It is important to note that HYSYS predicts only liquid- vapour surface tensions. A.4.7 Heat Capacity Heat Capacity is calculated using a rigorous Cv value whenever HYSYS can. The method used is given by the following equations: However, when ever this equation fails to provide an answer HYSYS will fall back to the semi-ideal Cp/Cv method by computing Cp/Cv as Cp/ (Cp-R), which is only approximate and valid for ideal gases. Examples of when HYSYS will use the ideal method are: • Equation (A.58) fails to return an answer • The stream has a solid phase • abs(dV/dP) < 1e-12 • Cp/Cv < 0.1or Cp/Cv > 20 - this is outside the range of applicability of the equation used so HYSYS falls back to the ideal method (A.57)σ Pc 2 3⁄ Tc 1 3⁄ Q 1 TR–( )a b= (A.58)Cp Cv– T– dV dT⁄( )2 dV dT⁄( )⁄⋅= Property Methods and Calculations A-55 A-55 A.5 Volumetric Flow Rate Calculations HYSYS has the ability to interpret and produce a wide assortment of flow rate data. It can accept several types of flow rate information for stream specifications as well as report back many different flow rates for streams, their phases and their components. One drawback of the large variety available is that it often leads to some confusion as to what exactly is being specified or reported, especially when volumetric flow rates are involved. In the following sections, the available flow rates are listed, each corresponding density basis is explained, and the actual formulation of the flow rate calculations is presented. For volumetric flow rate data that is not directly accepted as a stream specification, a final section is provided that outlines techniques to convert your input to mass flow rates. A.5.1 Available Flow Rates Many types of flow rates appear in HYSYS output. However, only a subset of these are available for stream specifFications. Flow Rates Reported In The Output The flow rate types available via our numerous reporting methods - property views, workbook, PFD, specsheets etc. are: • Molar Flow • Mass Flow • Std Ideal Liq Vol Flow • Liq Vol Flow @Std Cond • Actual Volume Flow • Std Gas Flow • Actual Gas Flow A-56 Volumetric Flow Rate Calculations A-56 Flow Rates Available For Specification You will find that the following flow rate types are available for stream specifications: • Molar Flows • Mass Flows • LiqVol Flows A.5.2 Liquid and Vapour Density Basis All calculations for volumetric stream flows are based on density. HYSYS utilizes the following density basis: Calculation of Standard and Actual Liquid Densities The Standard and Actual liquid densities are calculated rigorously at the appropriate T and P using the internal methods of the chosen property package. Flow rates based upon these densities automatically take into account any mixing effects exhibited by non-ideal systems. Thus, these volumetric flow rates may be considered as "real world". Calculation of Standard Ideal Liquid Mass Density Contrary to the rigorous densities, the Standard Ideal Liquid Mass density of a stream does not take into account any mixing effects due to its simplistic assumptions. Thus, flow rates that are based upon it will not account for mixing effects and are more empirical in nature. The calculation is as follows: The volumetric flow rate reference state is defined as 60°F and 1 atm when using Field units or 15°C and 1 atm when using SI units. Actual Densities are calculated at the stream Temperature and Pressure. Density Basis Description Std Ideal Liq Mass Density This is calculated based on ideal mixing of pure component ideal densities at 60°F. Liq Mass Density @Std Cond This is calculated rigorously at the standard reference state for volumetric flow rates. Actual Liquid Density This is calculated rigorously at the flowing conditions of the stream (i.e. at stream T and P). Standard Vapour Density This is determined directly from the Ideal Gas law. Actual Vapour Density This is calculated rigorously at the flowing conditions of the stream (i.e. at stream T and P). Property Methods and Calculations A-57 A-57 where: xi = molar fraction of component i ρi Ideal = pure component Ideal Liquid density HYSYS contains Ideal Liquid densities for all components in the Pure Component Library. These values have been determined in one of three ways, based on the characteristics of the component, as described below: Case 1 - For any component that is a liquid at 60°F and 1 atm, the data base contains the density of the component at 60°F and 1 atm. Case 2 - For any component that can be liquefied at 60°F and pressures greater than 1 atm, the data base contains the density of the component at 60°F and Saturation Pressure. Case 3 - For any component that is non-condensable at 60°F under any pressure, i.e. 60°F is greater than the critical temperature of the component, the data base contains GPA tabular values of the equivalent liquid density. These densities were experimentally determined by measuring the displacement of hydrocarbon liquids by dissolved non-condensable components. For all hypothetical components, the Standard Liquid density (Liquid Mass Density @Std Conditions) in the Base Properties will be used in the Ideal Liquid density (Std Ideal Liq Mass Density) calculation. If a density is not supplied, the HYSYS estimated liquid mass density (at standard conditions) will be used. Special treatment is given by the Oil Characterization feature to its pseudo components such that the ideal density calculated for its streams match the assay, bulk property, and flow rate data supplied in the Oil Characterization Environment. (A.59) Ideal DensityStream 1 xi ρi Ideal -------------∑ ---------------------= A-58 Volumetric Flow Rate Calculations A-58 A.5.3 Formulation of Flow Rate Calculations The various procedures used to calculate each of the available flow rates are detailed below, based on a known molar flow: Molar Flow Rate Mass Flow Std Ideal Liq Vol Flow This volumetric flow rate is calculated using the ideal density of the stream and thus is somewhat empirical in nature. Liq Vol Flow @Std Cond This volumetric flow rate is calculated using a rigorous density calculated at standard conditions, and will reflect non-ideal mixing effects. (A.60)Total Molar Flow Molar FlowStream= (A.61)Mass Flow Total Molar Flow MWStream×= Note that even if a stream is all vapour, it will still have a LiqVolume flow, based upon the stream’s Standard Ideal Liquid Mass density, whose calculation is detailed in the previous section. (A.62)LiqVolFlow Total Molar Flow MWStream× Ideal DensityStream --------------------------------------------------------------------------= (A.63)Std Liquid Volume Flow Molar Flow MW× Std Liq Density --------------------------------------------= Property Methods and Calculations A-59 A-59 Actual Volume Flow This volumetric flow rate is calculated using a rigorous liquid density calculation at the actual stream T and P conditions, and will reflect non-ideal mixing effects. Standard Gas Flow Standard gas flow is based on the molar volume of an ideal gas at standard conditions. It is a direct conversion from the stream’s molar flow rate, based on the following: • Ideal Gas at 60°F and 1 atm occupies 379.46 ft3/lbmole • Ideal Gas at 15°C and 1 atm occupies 23.644 m3/kgmole Actual Gas Flow This volumetric flow rate is calculated using a rigorous vapour density calculation at the actual stream T and P conditions, and will reflect non-ideal mixing and compressibility effects. A.5.4 Volumetric Flow Rates as Specifications If you require that the flow rate of your stream be specified based on actual density or standard density as opposed to Standard Ideal Mass Liquid density, you must utilize one of the following procedures: Liq Vol Flow @Std Cond 1. Specify the composition of your stream. 2. Use the standard ideal liquid mass density reported for the stream and calculate the corresponding mass flow rate either manually, or in the SpreadSheet. 3. Use this calculated mass flow as the specification for the stream. (A.64)Actual Volume Flow Molar Flow MW× Density --------------------------------------------= (A.65)Actual Gas Flow Molar Flow MW× Density --------------------------------------------= A-60 Flash Calculations A-60 Actual Liquid Volume Flow 1. Specify the composition and the flowing conditions (T and P) of your stream. 2. Use the density reported for the stream and calculate the corresponding mass flow rate either manually, or in our spreadsheet. 3. Use this calculated mass flow as the specification for the stream. A.6 Flash Calculations Rigorous three phase calculations are performed for all equations of state and activity models with the exception of Wilson’s equation, which only performs two phase vapour-liquid calculations. As with the Wilson Equation, the Amines and Steam property packages only support two phase equilibrium calculations. HYSYS uses built-in intelligence to determine when it can perform a flash calculation on a stream, and then what type of flash calculation needs to be performed on the stream. This is based completely on the degrees of freedom concept. Once the composition of a stream and two property variables are known, (vapour fraction, temperature, pressure, enthalpy or entropy) one of which must be either temperature or pressure, the thermodynamic state of the stream is defined. When HYSYS recognizes that a stream is thermodynamically defined, it will perform the correct flash automatically in the background. You never have to instruct HYSYS to perform a flash calculation. Property variables can either be specified by you or back-calculated from another unit operation. A specified variable is treated as an independent variable. All other stream properties are treated as dependent variables and are calculated by HYSYS. In this manner, HYSYS also recognizes when a stream has been overspecified. For example, if you specify three stream properties plus composition, HYSYS will print out a warning message that an inconsistency exists for that stream. This also applies to streams where an inconsistency has been created through HYSYS calculations. For example, if a stream Temperature and Pressure are specified in a Flowsheet, but HYSYS back-calculates a different temperature for that stream as a result of an enthalpy balance across a unit operation, HYSYS will generate an Inconsistency message. Specified variables can only be re-specified by you or via RECYCLE ADJUST, or SpreadSheet operations. They will not change through any heat or material balance calculations. If a flash calculation has been performed on a stream, HYSYS will know all the property values of that stream, i.e., thermodynamic, physical and transport properties. Property Methods and Calculations A-61 A-61 A.6.1 T-P Flash Calculation The independent variables for this type of flash calculation are the temperature and pressure of the system, while the dependent variables are the vapour fraction, enthalpy and entropy. With the equations of state and activity models, rigorous calculations are performed to determine the coexistence of immiscible liquid phases and the resulting component distributions by minimization of the Gibbs free energy term. For Vapour Pressure models or the Semi Empirical methods, the component distribution is based on the Kerosene solubility data (Figure 9A1.4 of the API Data Book). If the mixture is single-phase at the specified conditions, the property package calculates the isothermal compressibility (dv/dp) to determine if the fluid behaves as a liquid or vapour. Fluids in the dense-phase region are assigned the properties of the phase that best represents their current state. Note that material solids will appear in the liquid phase of two-phase mixtures, and in the heavy (aqueous/slurry) phase of three-phase systems. Therefore, when a separator is solved using a T-P flash, the vapour phase will be identical regardless of whether or not solids are present in the feed to the flash drum. A.6.2 Vapour Fraction Flash Vapour fraction and either temperature or pressure are the independent variables for this type of calculation. This class of calculation embodies all fixed quality points including bubble points (vapour pressure) and dew points. To perform bubble point calculation on a stream of known composition, simply specify the Vapour Fraction of the stream as 0.0 and define the temperature or pressure at which the calculation is desired. For a dew point calculation, simply specify the HYSYS will automatically perform the appropriate flash calculation when it recognizes that sufficient stream information is known. This information may have been either specified by the user or calculated by an operation. Depending on the known stream information, HYSYS will perform one of the following flashes: T-P, T-VF, T-H, T-S, P-VF, P-H, or P-S. See Section 1.4.5 - Stability Test Tab for options on how to instruct HYSYS to perform phase stability tests. Use caution in specifying solids with systems that are otherwise all vapour. Small amounts of non-solids may appear in the "liquid" phase. A-62 Flash Calculations A-62 Vapour Fraction of the stream as 1.0 and define the temperature or pressure at which the dew point calculation is desired. Like the other types of flash calculations, no initial estimates are required. Note that the vapour fraction is always shown in terms of the total number of moles. For instance, the vapour fraction (VF) represents the fraction of vapour in the stream, while the fraction, (1.0 - VF), represents all other phases in the stream (i.e. a single liquid, 2 liquids, a liquid and a solid). Dew Points Given a vapour fraction specification of 1.0 and either temperature or pressure, the property package will calculate the other dependent variable (P or T). If temperature is the second independent variable, HYSYS will calculate the dew point pressure. Likewise, if pressure is the independent variable, then the dew point temperature will be calculated. Retrograde dew points may be calculated by specifying a vapour fraction of -1.0. It is important to note that a dew point that is retrograde with respect to temperature can be normal with respect to pressure and vice versa. Bubble Points/Vapour Pressure A vapour fraction specification of 0.0 defines a bubble point calculation. Given this specification and either temperature or pressure, the property package will calculate the unknown T or P variable. As with the dew point calculation, if the temperature is known, HYSYS will calculate the bubble point pressure and conversely, given the pressure, HYSYS will calculate the bubble point temperature. For example, by fixing the temperature at 100°F, the resulting bubble point pressure is the true vapour pressure at 100°F. Quality Points Bubble and dew points are special cases of quality point calculations. Temperatures or pressures can be calculated for any vapour quality between 0.0 and 1.0 by specifying the desired vapour fraction and the corresponding independent variable. If HYSYS displays an error when calculating vapour fraction, then this means that the specified vapour fraction doesn't exist under the given conditions, i.e., the specified pressure is above the cricondenbar, or the given temperature lies to the right of the cricondentherm on a standard P-T envelope. All of the solids will appear in the liquid phase. Vapour pressure and bubble point pressure are synonymous. HYSYS will calculate the retrograde condition for the specified vapour quality if the vapour fraction is input as a negative number. Property Methods and Calculations A-63 A-63 A.6.3 Enthalpy Flash Given the enthalpy and either the temperature or pressure of a stream, the property package will calculate the unknown dependent variables. Although the enthalpy of a stream cannot be specified directly, it will often occur as the second property variable as a result of energy balances around unit operations such as valves, heat exchangers and mixers. If HYSYS responds with an error message, and cannot find the specified property (temperature or pressure), this probably means that an internally set temperature or pressure bound has been encountered. Since these bounds are set at quite large values, there is generally some erroneous input that is directly or indirectly causing the problem, such as an impossible heat exchange. A.6.4 Entropy Flash Given the entropy and either the temperature or pressure of a stream, the property package will calculate the unknown dependent variables. A.6.5 Handling of Water Water is handled differently depending on the correlation being used. The PR and PRSV equations have been enhanced to handle H2O rigorously whereas the semi-empirical and vapour pressure models treat H2O as a separate phase using steam table correlations. In these correlations, H2O is assumed to form an ideal, partially- miscible mixture with the hydrocarbons and its K value is computed from the relationship: where: p° = vapour pressure of H2O from Steam Tables P = system pressure xs = solubility of H2O in hydrocarbon liquid at saturation conditions. If a specified amount of energy is to be added to a stream, this may be accomplished by specifying the energy stream into either a COOLER/ HEATER or BALANCE operation. (A.66)Kω p° xsP( ) ------------= A-64 Flash Calculations A-64 The value for xs is estimated by using the solubility data for kerosene as shown in Figure 9A1.4 of the API Data Book19. This approach is generally adequate when working with heavy hydrocarbon systems. However, it is not recommended for gas systems. For three phase systems, only the PR and PRSV property package and Activity Models will allow components other than H2O in the second liquid phase. Special considerations are given when dealing with the solubilities of glycols and CH3OH. For acid gas systems, a temperature dependent interaction parameter was used to match the solubility of the acid component in the water phase. The PR equation considers the solubility of hydrocarbons in H2O, but this value may be somewhat low. The reason for this is that a significantly different interaction parameter must be supplied for cubic equations of state to match the composition of hydrocarbons in the water phase as opposed to the H2O composition in the hydrocarbon phase. For the PR equation of state, the latter case was assumed more critical. The second binary interaction parameter in the PRSV equation will allow for an improved solubility prediction in the alternate phase. With the activity coefficient models, the limited mutual solubility of H2O and hydrocarbons in each phase can be taken into account by implementing the insolubility option (please refer to Section A.2.2 - Activity Models). HYSYS will generate, upon request, interaction parameters for each activity model (with the exception of the Wilson equation) that have been fitted to match the solubility of H2O in the liquid hydrocarbon phase and hydrocarbons in the aqueous phase based on the solubility data referred to in that section. Note that the Peng-Robinson and SRK property packages will always force the water rich phase into the heavy liquid phase of a three phase stream. As such, the aqueous phase is always forced out of the bottom of a three phase separator, even if a light liquid phase (hydrocarbon rich) does not exist. Solids will always be carried in the second liquid phase. Property Methods and Calculations A-65 A-65 A.6.6 Solids HYSYS does not check for solid phase formation of pure components within the flash calculations, however, incipient solid formation conditions for CO2 and hydrates can be predicted with the Utility Package (for more information refer toChapter 8 - Utilities of the User’s Guide). Solid materials such as catalyst or coke can be handled as user-defined, solid type components. The HYSYS property package takes this type of component into account in the calculation of the following stream variables: stream total flow rate and composition (molar, mass and volume), vapour fraction, entropy, enthalpy, specific heat, density, molecular weight, compressibility factor, and the various critical properties. Transport properties are computed on a solids-free basis. Note that solids will always be carried in the second liquid phase, i.e., the water rich phase. Solids do not participate in vapour-liquid equilibrium (VLE) calculations. Their vapour pressure is taken as zero. However, since solids do have an enthalpy contribution, they will have an effect on heat balance calculations. Thus, while the results of an Temperature flash will be the same whether or not such components are present, an Enthalpy flash will be affected by the presence of solids. A solid material component is entered as a hypothetical component in HYSYS. See Chapter 2 - Hypotheticals for more information on Hypotheticals. A-66 Flash Calculations A-66 A.6.7 Stream Information When a flash calculation occurs for a stream, the information that is returned is dependent on the phases present within the stream. The following table shows the stream properties that will be calculated for each phase. Steam Property Applicable PhasesA Vapour Phase Mole Fraction F V L S Vapour Phase Mass Fraction F V L S Vapour Phase Volume Fraction F V L S Temperature F V L S Pressure F V L S Flow F V L S Mass Flow F V L S Liquid Volume Flow (Std, Ideal) F V L S Volume Flow F V L S Std. Gas Flow F V L S Std. Volume Flow F L S Energy F V L S Molar Enthalpy F V L S Mass Enthalpy F V L S Molar Entropy F V L S Mass Entropy F V L S Molar Volume F V L S Molar Density F V L S Mass Density F V L S Std. Liquid Mass Density FD L S Molar Heat Capacity F V L S Mass Heat Capacity F V L S CP/CV F V L S Thermal Conductivity FB,D V L Viscosity FB,D V L Kinematic Viscosity FB,D V L Surface Tension FB L Molecular Weight F V L S Z Factor FB V L S Air SG FB V Watson (UOP) K Value F V L S Component Mole Fraction F V L S Component Mass Fraction F V L S Property Methods and Calculations A-67 A-67 AStream phases: BPhysical property queries are allowed on the feed phase of single phase streams. CPhysical property queries are allowed on the feed phase only for streams containing vapour and/or liquid phases. DPhysical property queries are allowed on the feed phase of liquid streams with more than one liquid phase. A.7 References 1 Peng, D. Y. and Robinson, D. B., "A Two Constant Equation of State", I.E.C. Fundamentals, 15, pp. 59-64 (1976). 2 Soave, G., Chem Engr. Sci., 27, No. 6, p. 1197 (1972). 3 Knapp, H., et al., "Vapor-Liquid Equilibria for Mixtures of Low Boiling Substances", Chemistry Data Series Vol. VI, DECHEMA, 1989. 4 Kabadi, V.N., and Danner, R.P. A Modified Soave-Redlich-Kwong Equation of State for Water-Hydrocarbon Phase Equilibria, Ind. Eng. Chem. Process Des. Dev. 1985, Volume 24, No. 3, pp 537-541. Component Volume Fraction F V L S Component Molar Flow F V L S Component Mass Flow F V L S Component Volume Flow F V L S K Value (y/x) Lower Heating Value Mass Lower Heating Value Molar Liquid Fraction F V L S Molar Light Liquid Fraction F V L S Molar Heavy Liquid Fraction F V L S Molar Heat of Vapourization FC V L Mass Heat of Vapourization FC V L Partial Pressure of CO2 F V L S Steam Property Applicable PhasesA F Feed V Vapour L Liquid S Solid A-68 References A-68 5 Stryjek, R., Vera, J.H., J. Can. Chem. Eng., 64, p. 334, April 1986. 6 API Publication 955, A New Correlation of NH3, CO2 and H2S Volatility Data From Aqueous Sour Water Systems, March 1978. 7 Zudkevitch, D., Joffee, J. "Correlation and Prediction of Vapor-Liquid Equilibria with the Redlich-Kwong Equation of State", AIChE Journal, Volume 16, No. 1, January pp. 112-119. 8 Reid, C.R., Prausnitz, J.M. and Sherwood, T.K., "The Properties of Gases and Liquids", McGraw-Hill Book Company, 1977. 9 Prausnitz, J.M., Lichtenthaler, R.N., Azevedo, E.G., "Molecular Thermodynamics of Fluid Phase Equilibria", 2nd. Ed., McGraw- Hill, Inc. 1986. 10Chao, K. D. and Seader, J. D., A.I.Ch.E. Journal, pp. 598-605, December 1961. 11Grayson, H. G. and Streed, G. W., "Vapour-Liquid Equilibria for High Temperature, High Pressure Systems", 6th World Petroleum Congress, West Germany, June 1963. 12Jacobsen, R.T and Stewart, R.B., 1973. "Thermodynamic Properties of Nitrogen Including Liquid and Vapour Phases from 63 K to 2000K with Pressure to 10 000 Bar." J. Phys. Chem. Reference Data, 2: 757- 790. 13Hankinson, R.W. and Thompson, G.H., A.I.Ch.E. Journal, 25, No. 4, p. 653 (1979). 14Ely, J.F. and Hanley, H.J.M., "A Computer Program for the Prediction of Viscosity and Thermal Conductivity in Hydrocarbon Mixtures", NBS Technical Note 1039. 15Twu, C.H., I.E.C. Proc Des & Dev, 24, p. 1287 (1985). 16Reid, R.C., Prausnitz, J.M., Poling, B.E., "The Properties of Gases & Liquids", McGraw-Hill, Inc., 1987. 17Woelflin, W., "Viscosity of Crude-Oil Emulsions", presented at the spring meeting, Pacific Coast District, Division of Production, Los Angeles, Calif., Mar. 10, 1942. 18Gambill, W.R., Chem. Eng., March 9, 1959. 19API Technical Data Book, Petroleum Refining, Fig. 9A1.4, p. 9-15, 5th Edition (1978). Property Methods and Calculations A-69 A-69 20Keenan, J. H. and Keyes, F. G., Thermodynamic Properties of Steam, Wiley and Sons (1959). 21Perry, R. H.; Green, D. W.; “Perry’s Chemical Engineers’ Handbook Sixth Edition”, McGraw-Hill Inc., (1984). 22Passut, C. A.; Danner, R. P., “Development of a Four-Parameter Corresponding States Method: Vapour Pressure Prediction”, Thermodynamics - Data and Correlations, AIChE Symposium Series; p. 30-36, No. 140, Vol. 70. A-70 References A-70 Petroleum Methods/Correlations B-1 B-1 B Petroleum Methods/ Correlations B.1 Characterization Method............................................................................. 3 B.1.1 Generate a Full Set of Working Curves ................................................... 3 B.1.2 Light Ends Analysis ................................................................................. 4 B.1.3 Auto Calculate Light Ends ....................................................................... 7 B.1.4 Determine TBP Cutpoint Temperatures................................................... 7 B.1.5 Graphically Determine Component Properties ........................................ 8 B.1.6 Calculate Component Critical Properties................................................. 9 B.1.7 Correlations ............................................................................................. 9
缩略图:
当前页面二维码



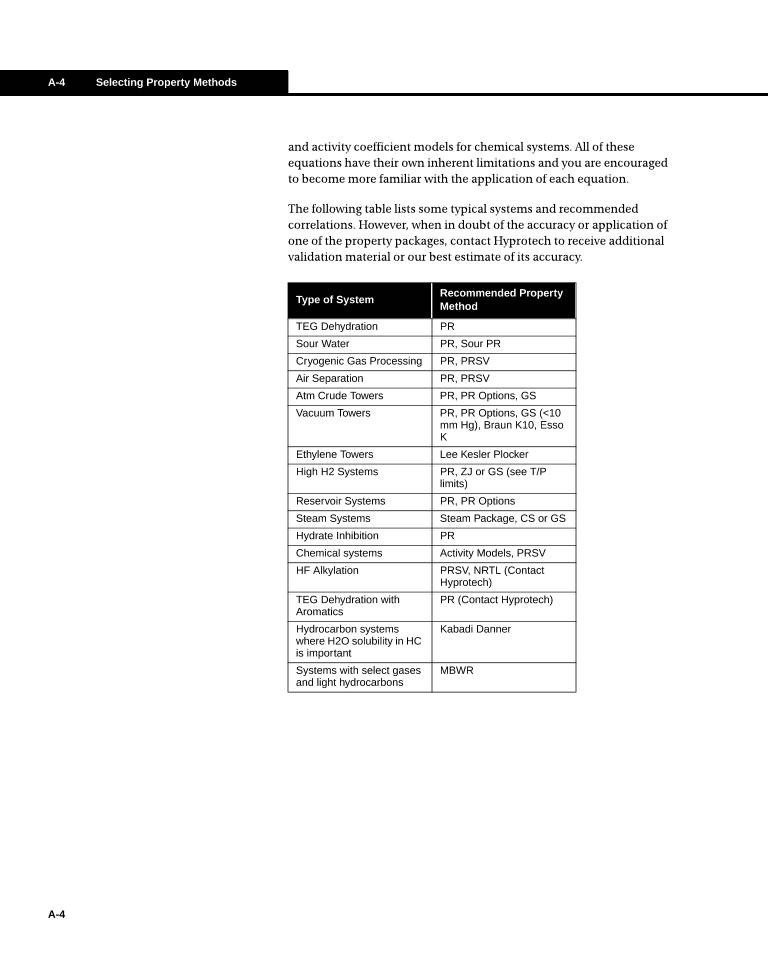



 工程招标采购
工程招标采购 搞笑表情
搞笑表情 微信头像
微信头像 美女图片
美女图片 APP小游戏
APP小游戏 PPT模板
PPT模板